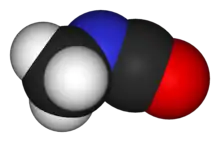
Methyl isocyanate
Methyl isocyanate (MIC) is an organic compound with the molecular formula CH3NCO. Synonyms are isocyanatomethane and methyl carbylamine. Methyl isocyanate is an intermediate chemical in the production of carbamate pesticides (such as carbaryl, carbofuran, methomyl, and aldicarb). It has also been used in the production of rubbers and adhesives. As an extremely toxic and irritating compound, it is very hazardous to human health. It was the principal toxicant involved in the infamous Bhopal gas disaster, which killed 2,259 people initially and officially 20,000 people in total.[5][6][7][8][9][10][11] It is also a very potent lachrymatory agent.
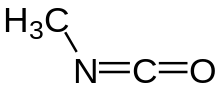 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Isocyanatomethane | |
| Other names
Methyl carbylamine MIC | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.009.879 |
IUPHAR/BPS |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H3NO | |
| Molar mass | 57.051 g/mol |
| Appearance | Colorless liquid |
| Odor | Sharp, pungent odor[1] |
| Density | 0.9230 g/cm3 at 27 °C |
| Melting point | −45 °C (−49 °F; 228 K)[2] |
| Boiling point | 38.3 °C (100.9 °F; 311.4 K)[2] |
| 10% (15°C)[1] | |
| Vapor pressure | 57.7 kPa |
| Structure | |
Dipole moment |
2.8 D |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
−92.0 kJ·mol−1[2] |
| Hazards | |
| GHS labelling: | |
     | |
Hazard statements |
H225, H300, H311, H315, H317, H318, H330, H334, H335, H361d |
Precautionary statements |
P201, P202, P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P272, P280, P281, P284, P285, P301+P310, P302+P352, P303+P361+P353, P304+P340, P304+P341, P305+P351+P338, P308+P313, P310, P312, P320, P321, P322, P330, P332+P313, P333+P313, P342+P311, P361, P362, P363, P370+P378, P403+P233, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | |
| Flash point | −7 °C (19 °F; 266 K) |
Autoignition temperature |
534 °C (993 °F; 807 K) |
| Explosive limits | 5.3–26%[2] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
120 mg/kg (oral, mouse) 51.5 mg/kg (oral, rat)[3] |
LC50 (median concentration) |
6.1 ppm (rat, 6 hr) 12.2 ppm (mouse, 6 hr) 5.4 ppm (guinea pig, 6 hr) 21 ppm (rat, 2 hr)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 0.02 ppm (0.05 mg/m3) [skin][1] |
REL (Recommended) |
TWA 0.02 ppm (0.05 mg/m3) [skin][1] |
IDLH (Immediate danger) |
3 ppm[1] |
| Related compounds | |
Related compounds |
Methyl isothiocyanate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Physical properties
Methyl isocyanate is a colorless, poisonous, lachrymatory (tearing agent), flammable liquid.[12] It is soluble in water to 6–10 parts per 100 parts, but it also reacts with water (see Reactions below).
Manufacture
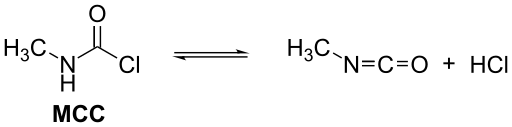
Methyl isocyanate is usually manufactured by the reaction of monomethylamine and phosgene. For large scale production, it is advantageous to combine these reactants at higher temperature in the gas phase. A mixture of methyl isocyanate and two moles of hydrogen chloride is formed, but N-methylcarbamoyl chloride (MCC) forms as the mixture is condensed, leaving one mole of hydrogen chloride as a gas.
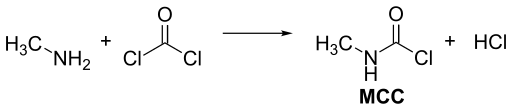
The methyl isocyanate is obtained by treating the MCC with a tertiary amine, such as N,N-dimethylaniline, or with pyridine[13] or by separating it by using distillation techniques.[14]
Methyl isocyanate is also manufactured from N-methylformamide and air. In the latter process, it is immediately consumed in a closed-loop process to make methomyl.[15] Other manufacturing methods have been reported.[16][17]
Reactions
Methyl isocyanate reacts readily with many substances that contain N-H or O-H groups. With water, it forms 1,3-dimethylurea and carbon dioxide with the evolution of heat (1358.5 joules, or 325 calories, per gram of MIC):

At 25 °C, in excess water, half of the MIC is consumed in 9 min.;[18] if the heat is not efficiently removed from the reacting mixture, the rate of the reaction will increase and rapidly cause the MIC to boil. Such a reaction triggered the Bhopal disaster after that water was accidentally introduced in a MIC storage tank during cleaning operation of an adjacent pipe without closing the proper isolation valve of the reservoir. The consequence of the out of control exothermic process was a runaway reaction and the direct release of 42 tons of MIC to the atmosphere.
If MIC is in excess, 1,3,5-trimethylbiuret is formed along with carbon dioxide.[12] Alcohols and phenols, which contain an O-H group, react slowly with MIC, but the reaction can be catalyzed by trialkylamines or dialkyltin dicarboxylate. Oximes, hydroxylamines, and enols also react with MIC to form methylcarbamates.[12] These reactions produce the products described below (Uses).
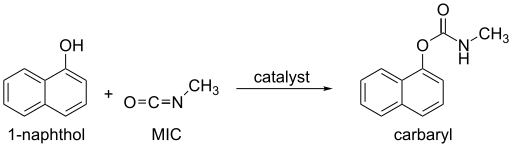
Ammonia, primary, and secondary amines rapidly react with MIC to form substituted ureas. Other N-H compounds, such as amides and ureas, react much more slowly with MIC.[19]
It also reacts with itself to form a trimer or higher molecular weight polymers. In the presence of catalysts, MIC reacts with itself to form a solid trimer, trimethyl isocyanurate, or a higher molecular weight polymer:
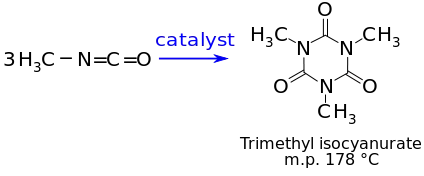
Sodium methoxide, triethyl phosphine, ferric chloride and certain other metal compounds catalyze the formation of the MIC-trimer, while the high-molecular-weight polymer formation is catalyzed by certain trialkylamines. Since the formation of the MIC trimer is exothermic (1246 joules, or 298 calories, per gram of MIC), the reaction can lead to violent boiling of the MIC. The high-molecular-weight polymer hydrolyzes in hot water to form the trimethyl isocyanurate. Since catalytic metal salts can be formed from impurities in commercial grade MIC and steel, this product must not be stored in steel drums or tanks.[12]
Toxicity
Methyl isocyanate is extremely toxic. There is no known antidote. The threshold limit value set by the American Conference of Governmental Industrial Hygienists is 0.02 ppm. MIC is toxic by inhalation, ingestion and contact in quantities as low as 0.4 ppm. Exposure symptoms include coughing, chest pain, dyspnea, asthma, irritation of the eyes, nose and throat, as well as skin damage. Higher levels of exposure, over 21 ppm, can result in pulmonary or lung edema, emphysema and hemorrhages, bronchial pneumonia and death. Although the odor of methyl isocyanate cannot be detected at 5 ppm by most people, its potent lachrymal properties provide an excellent warning of its presence (at a concentration of 2–4 parts per million (ppm) subject's eyes are irritated, while at 21 ppm, subjects could not tolerate the presence of methyl isocyanate in air).[20]
Proper care must be taken to store methyl isocyanate because of its ease of exothermically polymerizing (see Reactions) and its similar sensitivity to water. Only stainless steel or glass containers may be safely used; the MIC must be stored at temperatures below 40 °C (104 °F) and preferably at 4 °C (39 °F).
The toxic effect of the compound was apparent in the 1984 Bhopal disaster, when around 42,000 kilograms (93,000 lb) of methyl isocyanate and other gases were released from the underground reservoirs of the Union Carbide India Limited (UCIL) factory, over a populated area on 3 December 1984, killing about 3,500 people immediately and 15,000 more over the next several years.[21]
During structural fires, natural materials can contribute to releasing isocyanates including methyl isocyanate. [22]
Mechanism of action
Until recent decades, the mechanism of methyl isocyanate toxicity in humans was largely unknown or unclear.[23][24] Methyl isocyanate and other isocyanates are electrophiles and are currently thought to cause toxicity by the alkylation of biomolecules.[25] The mechanism of methyl isocyanate was previously suspected to be the carbamylation of hemoglobin which would interfere with its oxygen binding capability causing hypoxia. However, experiments showed that when rats and guinea pigs were exposed to methyl isocyanate at concentrations above the median lethal concentration (LC50, the concentration sufficient to kill 50% of the tested population), only 2% of hemoglobin molecules were carbamylated, suggesting that this is probably not the mechanism of toxicity.[26][27]
Extraterrestrial occurrence

On 30 July 2015, scientists reported that upon the first touchdown of the Philae lander on comet 67/P's surface, measurements by the COSAC and Ptolemy instruments revealed sixteen organic compounds, four of which were seen for the first time on a comet, including acetamide, acetone, methyl isocyanate and propionaldehyde.[29][30][31]
In 2017, two teams of astronomers using the Atacama Large Millimeter Array (ALMA) interferometer made of 66 radio telescopes in the Atacama Desert (northern Chile) have discovered the presence of MIC around young Sun-like stars.[28]
MIC is considered a prebiotic molecule as explained by the discoverers of the ALMA findings in IRAS 16293-2422, a multiple system of very young stars: "This family of organic molecules is involved in the synthesis of peptides and amino acids, which, in the form of proteins, are the biological basis for life as we know it".[28]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0423". National Institute for Occupational Safety and Health (NIOSH).
- Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0487-3.
- "Methyl isocyanate". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- "NFPA Hazard Rating Information for Common Chemicals". nmsu.edu. Archived from the original on 17 February 2015. Retrieved 10 June 2021.
- Methyl Isocyanate. Union Carbide F-41443A – 7/76. Union Carbide Corporation, New York 1976
- Operating Manual Part II. Methyl Isocyanate Unit. Union Carbide India Limited, Agricultural Products Division, 1979
- Broughton E (May 2005). "The Bhopal disaster and its aftermath: a review". Environmental Health. 4 (1): 6. doi:10.1186/1476-069X-4-6. PMC 1142333. PMID 15882472.
- Eckerman I (2001). "Chemical Industry and Public Health — Bhopal as an example" (PDF). MPH. Göteborg, Sweden: Nordic School of Public Health. 2001 (24). ISSN 1104-5701. Archived (PDF) from the original on 30 October 2012.
- Eckerman I (2004). The Bhopal Saga - Causes and Consequences of the World's Largest Industrial Disaster. India: Universities Press. ISBN 81-7371-515-7. Archived from the original on 10 June 2007.
- Rosenberg J. "At 1984 - Huge Poison Gas Leak in Bhopal, India". About.com. Archived from the original on 2 December 2007. Retrieved 10 July 2008.
- Eckerman I (2013). "Bhopal Gas Catastrophe 1984: Causes and Consequences". Reference Module in Earth Systems and Environmental Sciences. Elsevier. pp. 272–287. doi:10.1016/B978-0-12-409548-9.01903-5. ISBN 978-0-12-409548-9.
- Union Carbide Corporation "Methyl Isocyanate" Product Information Publication, F-41443, November 1967.
- US patent 2480088, Slocombe, R. J.; Hardy, E. E., "Process of Producing Carbamyl Chlorides", issued 1949-08-23, assigned to Monsanto
- FR patent 1400863, Merz, W., "Procédé et dispositif de préparation d'isocyanates d'alkyle", issued 1965-05-28, assigned to Bayer
- Chemical Week, "A fleeting existence for toxic-gas molecules" p. 9, 12 June 1985.
- DE patent 2828259, Giesselmann, G.; Guenther, K.; Fuenten, W., "Verfahren zur Herstellung von Methyl Isocyanate", issued 1980-01-10, assigned to Degussa
- "A safer method for making carbamates". Chemical Week. 1985b (20): 136. 1985.
- Castro EA, Moodie RB, Sansom PJ (1985). "The kinetics of hydrolysis of methyl and phenyl isocyanates". Journal of the Chemical Society, Perkin Transactions 2. 1985 (5): 737–742. doi:10.1039/P29850000737.
- March J (1985). Advanced Organic Chemistry (3rd ed.). New York: John Wiley & Sons. p. 802.
- Kimmerle G, Eben A (1964). "Zur Toxizität von Methylisocyanat und dessen quantitativer Bestimmung in der Luft". Archiv für Toxikologie. 20 (4): 235–241. doi:10.1007/bf00577897. S2CID 21422558.
- "Bhopal trial: Eight convicted over India gas disaster". BBC News. 7 June 2010. Archived from the original on 7 June 2010. Retrieved 7 June 2010.
- Dzhordzhio Naldzhiev, Matija Strlic; Polyurethane insulation and household products - A systematic review of their impact on indoor environmental quality, [Building and Environment https://www.sciencedirect.com/journal/building-and-environment], 2020
- Mehta PS, Mehta AS, Mehta SJ, Makhijani AB (December 1990). "Bhopal tragedy's health effects. A review of methyl isocyanate toxicity". JAMA. 264 (21): 2781–7. doi:10.1001/jama.1990.03450210081037. PMID 2232065.
- Varma DR (June 1987). "Epidemiological and experimental studies on the effects of methyl isocyanate on the course of pregnancy". Environmental Health Perspectives. 72: 153–7. doi:10.1289/ehp.8772153. PMC 1474644. PMID 3622430.
- Bessac, B. F.; Jordt, S.-E. (1 July 2010). "Sensory Detection and Responses to Toxic Gases: Mechanisms, Health Effects, and Countermeasures". Proceedings of the American Thoracic Society. 7 (4): 269–277. doi:10.1513/pats.201001-004sm. ISSN 1546-3222. PMC 3136963. PMID 20601631.
- Varma, Daya R.; Guest, Ian (1993). "The Bhopal accident and methyl isocyanate toxicity". Journal of Toxicology and Environmental Health. 40 (4): 513–529. doi:10.1080/15287399309531816. ISSN 0098-4108. PMID 8277516.
- Ramachandran, P.K.; Gandhe, B.R.; Venkateswaran, K.S.; Kaushik, M.P.; Vijayaraghavan, R.; Agarwal, G.S.; Gopalan, N.; Suryanarayana, M.V.S.; Shinde, S.K.; Sriramachari, S. (1988). "Gas chromatographic studies of the carbamylation of haemoglobin by methyl isocyanate in rats and rabbits". Journal of Chromatography B: Biomedical Sciences and Applications. 426 (2): 239–247. doi:10.1016/s0378-4347(00)81952-0. ISSN 0378-4347. PMID 3392138.
- "ALMA Finds Ingredient of Life Around Infant Sun-like Stars". www.eso.org. Archived from the original on 8 June 2017. Retrieved 8 June 2017.
- Jordans F (30 July 2015). "Philae probe finds evidence that comets can be cosmic labs". The Washington Post. Associated Press. Archived from the original on 23 December 2018. Retrieved 30 July 2015.
- "Science on the Surface of a Comet". European Space Agency. 30 July 2015. Archived from the original on 2 August 2015. Retrieved 30 July 2015.
- Bibring JP, Taylor MG, Alexander C, Auster U, Biele J, Finzi AE, et al. (July 2015). "Philae's first look. Philae's First Days on the Comet. Introduction". Science. 349 (6247): 493. Bibcode:2015Sci...349..493B. doi:10.1126/science.aac5116. PMID 26228139.
External links
- NIOSH Safety and Health Topic: Isocyanates, from the website of the National Institute for Occupational Safety and Health (NIOSH).
- U.S. National Library of Medicine: Hazardous Substances Databank – Methyl isocyanate
