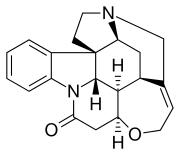
Strychnine
Strychnine (/ˈstrɪkniːn, -nɪn/, STRIK-neen, -nin, US chiefly /-naɪn/ -nyne)[5][6] is a highly toxic, colorless, bitter, crystalline alkaloid used as a pesticide, particularly for killing small vertebrates such as birds and rodents. Strychnine, when inhaled, swallowed, or absorbed through the eyes or mouth, causes poisoning which results in muscular convulsions and eventually death through asphyxia.[7] While it is no longer used medicinally, it was used historically in small doses to strengthen muscle contractions, such as a heart and bowel stimulant[8] and performance-enhancing drug. The most common source is from the seeds of the Strychnos nux-vomica tree.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Strychnidin-10-one[1] | |
| Preferred IUPAC name
(4aR,5aS,8aR,13aS,15aS,15bR)-4a,5,5a,7,8,13a,15,15a,15b,16-decahydro-2H-4,6-methanoindolo[3,2,1-ij]oxepino[2,3,4-de]pyrrolo[2,3-h]quinolin-14-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.290 |
IUPHAR/BPS |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII |
|
| UN number | 1692 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H22N2O2 | |
| Molar mass | 334.419 g·mol−1 |
| Appearance | White or translucent crystal or crystalline powder; Bitter tasting |
| Odor | Odorless |
| Density | 1.36 g cm−3 |
| Melting point | 270 °C; 518 °F; 543 K |
| Boiling point | 284 to 286 °C; 543 to 547 °F; 557 to 559 K |
| 0.02% (20°C)[2] | |
| Acidity (pKa) | 8.25[3] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Extremely toxic |
| GHS labelling: | |
  | |
| Danger | |
Hazard statements |
H300, H310, H330, H410 |
Precautionary statements |
P260, P264, P273, P280, P284, P301+P310 |
| NFPA 704 (fire diamond) | |
| Flash point | Non flammable |
Autoignition temperature |
Non flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
0.5 mg/kg (dog, oral) 0.5 mg/kg (cat, oral) 2 mg/kg (mouse, oral) 16 mg/kg (rat, oral) 2.35 mg/kg (rat, oral)[4] |
LDLo (lowest published) |
0.6 mg/kg (rabbit, oral)[4] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 0.15 mg/m3[2] |
REL (Recommended) |
TWA 0.15 mg/m3[2] |
IDLH (Immediate danger) |
3 mg/m3[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Biosynthesis
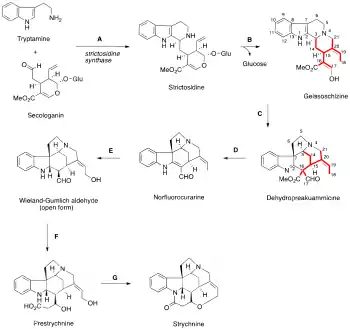
Strychnine is a terpene indole alkaloid belonging to the Strychnos family of Corynanthe alkaloids, and it is derived from tryptamine and secologanin.[9][10] The biosynthesis of strychine has been solved in 2022[11] The enzyme, strictosidine synthase, catalyzes the condensation of tryptamine and secologanin, followed by a Pictet-Spengler reaction to form strictosidine.[12] Many steps have been inferred by isolation of intermediates from Strychnos nux-vomica.[13] The next step is hydrolysis of the acetal, which opens the ring by elimination of glucose (O-Glu) and provides a reactive aldehyde. The nascent aldehyde is then attacked by a secondary amine to afford geissoschizine, a common intermediate of many related compounds in the Strychnos family.[9]
A reverse Pictet-Spengler reaction cleaves the C2–C3 bond, while subsequently forming the C3–C7 bond via a 1,2-alkyl migration, an oxidation from a cytochrome P450 enzyme to a spiro-oxindole, nucleophilic attack from the enol at C16, and elimination of oxygen forms the C2–C16 bond to provide dehydropreakuammicine.[14] Hydrolysis of the methyl ester and decarboxylation leads to norfluorocurarine. Stereospecific reduction of the endocyclic double bond by NADPH and hydroxylation provides the Wieland-Gumlich aldehyde, which was first isolated by Heimberger and Scott in 1973, although previously synthesized by Wieland and Gumlich in 1932.[13][15] To elongate the appendage by 2 carbons, acetyl-CoA is added to the aldehyde in an aldol reaction to afford prestrychnine. Strychnine is then formed by a facile addition of the amine with the carboxylic acid or its activated CoA thioester, followed by ring-closure via displacement of an activated alcohol.
Chemical synthesis
As early researchers have noted, the strychnine molecular structure, with its specific array of rings, stereocenters, and nitrogen functional groups, is a complex synthetic target, and has stimulated interest for that reason and for interest in the structure-activity relationships underlying its pharmacologic activities.[16] An early synthetic chemist targeting strychnine, R.B. Woodward, quoted the chemist who determined its structure through chemical decomposition and related physical studies as saying that "for its molecular size it is the most complex organic substance known" (attributed to Sir Robert Robinson).[17]
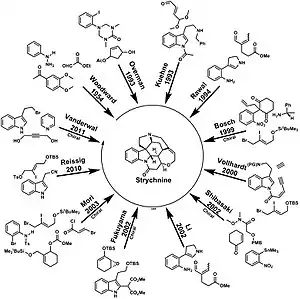
The first total synthesis of strychnine was reported by the research group of R. B. Woodward in 1954, and is considered a classic in this field.[18][9] The Woodward account published in 1954 was very brief (3 pp.),[19] but was followed by a 42-page report in 1963.[20] The molecule has since received continuing wide attention in the years since for the challenges to synthetic organic strategy and tactics presented by its complexity; its synthesis has been targeted and its stereocontrolled preparation independently achieved by more than a dozen research groups since the first success (see main strychnine total synthesis article).
Mechanism of action
Strychnine is a neurotoxin which acts as an antagonist of glycine and acetylcholine receptors. It primarily affects the motor nerve fibers in the spinal cord which control muscle contraction. An impulse is triggered at one end of a nerve cell by the binding of neurotransmitters to the receptors. In the presence of an inhibitory neurotransmitter, such as glycine, a greater quantity of excitatory neurotransmitters must bind to receptors before an action potential is generated. Glycine acts primarily as an agonist of the glycine receptor, which is a ligand-gated chloride channel in neurons located in the spinal cord and in the brain. This chloride channel allows the negatively charged chloride ions into the neuron, causing a hyperpolarization which pushes the membrane potential further from threshold. Strychnine is an antagonist of glycine; it binds noncovalently to the same receptor, preventing the inhibitory effects of glycine on the postsynaptic neuron. Therefore, action potentials are triggered with lower levels of excitatory neurotransmitters. When the inhibitory signals are prevented, the motor neurons are more easily activated and the victim has spastic muscle contractions, resulting in death by asphyxiation.[7][21] Strychnine binds the Aplysia californica acetylcholine binding protein (a homolog of nicotinic receptors) with high affinity but low specificity, and does so in multiple conformations.[22]
Toxicity
In high doses, strychnine is very toxic to humans (minimum lethal oral dose in adults is 30–120 mg) and many other animals (oral LD50 = 16 mg/kg in rats, 2 mg/kg in mice),[23] and poisoning by inhalation, swallowing, or absorption through eyes or mouth can be fatal. S. nux-vomica seeds are generally effective as a poison only when they are crushed or chewed before swallowing because the pericarp is quite hard and indigestible; poisoning symptoms may therefore not appear if the seeds are ingested whole.
Animal toxicity
Strychnine poisoning in animals usually occurs from ingestion of baits designed for use against gophers, moles, and coyotes. Strychnine is also used as a rodenticide, but is not specific to such unwanted pests and may kill other small animals.[24] In the United States, most baits containing strychnine have been replaced with zinc phosphide baits since 1990. In the European Union, rodenticides with strychnine are forbidden since 2006. Some animals are immune to strychnine, usually these are species such as fruit bats that have evolved resistance to poisonous alkaloids in the fruit they eat. The drugstore beetle has a symbiotic gut yeast that allows it to digest pure strychnine.
Strychnine toxicity in rats is dependent on sex. It is more toxic to females than to males when administered via subcutaneous injection or intraperitoneal injection. Differences are due to higher rates of metabolism by male rat liver microsomes. Dogs and cats are more susceptible among domestic animals, pigs are believed to be as susceptible as dogs, and horses are able to tolerate relatively large amounts of strychnine. Birds affected by strychnine poisoning exhibit wing droop, salivation, tremors, muscle tenseness, and convulsions. Death occurs as a result of respiratory arrest. The clinical signs of strychnine poisoning relate to its effects on the central nervous system. The first clinical signs of poisoning include nervousness, restlessness, twitching of the muscles, and stiffness of the neck. As the poisoning progresses, the muscular twitching becomes more pronounced and convulsions suddenly appear in all the skeletal muscles. The limbs are extended and the neck is curved to opisthotonus. The pupils are widely dilated. As death approaches, the convulsions follow one another with increased rapidity, severity, and duration. Death results from asphyxia due to prolonged paralysis of the respiratory muscles. Following the ingestion of strychnine, symptoms of poisoning usually appear within 15 to 60 min. The LD50-values for strychnine in animals are listed below in table 1.
| The LD50 values for strychnine in animals | ||
|---|---|---|
| Organism | Route | LD50 (mg/kg) |
| Bird-wild[25] | Oral | 16 |
| Cat[26] | Intravenous | 0.33 |
| Cat[27] | Oral | 0.5 |
| Dog[28] | Intravenous | 0.8 |
| Dog[26] | Subcutaneous | 0.35 |
| Dog[27] | Oral | 0.5 |
| Duck[25] | Oral | 3.0 |
| Mouse[29] | Intraperitoneal | 0.98 |
| Mouse[30] | Intravenous | 0.41 |
| Mouse[31] | Oral | 2.0 |
| Mouse[32] | Parenteral | 1.06 |
| Mouse[33] | Subcutaneous | 0.47 |
| Pigeon[25] | Oral | 21.0 |
| Quail[25] | Oral | 23.0 |
| Rabbit[28] | Intravenous | 0.4 |
| Rabbit[26] | Oral | 0.6 |
| Rat[34] | Oral | 16.0 |
| Rat[35] | Intravenous | 2.35 |
Human toxicity

After injection, inhalation, or ingestion, the first symptoms to appear are generalized muscle spasms. They appear very quickly after inhalation or injection – within as few as five minutes – and take somewhat longer to manifest after ingestion, typically approximately 15 minutes. With a very high dose, the onset of respiratory failure and brain death can occur in 15 to 30 minutes. If a lower dose is ingested, other symptoms begin to develop, including seizures, cramping, stiffness,[36] hypervigilance, and agitation.[37] Seizures caused by strychnine poisoning can start as early as 15 minutes after exposure and last 12–24 hours. They are often triggered by sights, sounds, or touch and can cause other adverse symptoms, including hyperthermia, rhabdomyolysis, myoglobinuric kidney failure, metabolic acidosis, and respiratory acidosis. During seizures, mydriasis (abnormal dilation), exophthalmos (protrusion of the eyes), and nystagmus (involuntary eye movements) may occur.[24]
As strychnine poisoning progresses, tachycardia (rapid heart beat), hypertension (high blood pressure), tachypnea (rapid breathing), cyanosis (blue discoloration), diaphoresis (sweating), water-electrolyte imbalance, leukocytosis (high number of white blood cells), trismus (lockjaw), risus sardonicus (spasm of the facial muscles), and opisthotonus (dramatic spasm of the back muscles, causing arching of the back and neck) can occur. In rare cases, the affected person may experience nausea or vomiting.[24]
The proximate cause of death in strychnine poisoning can be cardiac arrest, respiratory failure, multiple organ failure, or brain damage.[24]
The minimum lethal dose values estimated from different cases of strychnine poisoning are listed below in table 2.
| Minimum lethal dose estimates for strychnine in humans | ||
|---|---|---|
| Route | Dose (mg) | |
| Human[38][39] | Oral | 100–120 |
| Human[40] | Oral | 30–60 |
| Human (child)[41][42] | Oral | 15 |
| Human (adult)[43] | Oral | 50–100 |
| Human (adult)[42] | Oral | 30–100 |
| Human[44] | Intravenously | 5–10 (approximate) |
For occupational exposures to strychnine, the Occupational Safety and Health Administration and the National Institute for Occupational Safety and Health have set exposure limits at 0.15 mg/m3 over an 8-hour work day.[2]
Because strychnine produces some of the most dramatic and painful symptoms of any known toxic reaction, strychnine poisoning is often portrayed in literature and film including authors Agatha Christie and Arthur Conan Doyle.[45]
Pharmacokinetics
Absorption
Strychnine may be introduced into the body orally, by inhalation, or by injection. It is a potently bitter substance, and in humans has been shown to activate bitter taste receptors TAS2R10 and TAS2R46.[46][47][48] Strychnine is rapidly absorbed from the gastrointestinal tract.[49]
Distribution
Strychnine is transported by plasma and erythrocytes. Due to slight protein binding, strychnine leaves the bloodstream quickly and distributes to the tissues. Approximately 50% of the ingested dose can enter the tissues in 5 minutes. Also within a few minutes of ingestion, strychnine can be detected in the urine. Little difference was noted between oral and intramuscular administration of strychnine in a 4 mg dose.[50] In persons killed by strychnine, the highest concentrations are found in the blood, liver, kidney and stomach wall. The usual fatal dose is 60–100 mg strychnine and is fatal after a period of 1–2 hours, though lethal doses vary depending on the individual.
Metabolism
Strychnine is rapidly metabolized by the liver microsomal enzyme system requiring NADPH and O2. Strychnine competes with the inhibitory neurotransmitter glycine resulting in an excitatory state. However, the toxicokinetics after overdose have not been well described. In most severe cases of strychnine poisoning, the patient dies before reaching the hospital. The biological half-life of strychnine is about 10 hours. This half-life suggests that normal hepatic function can efficiently degrade strychnine even when the quantity ingested is high enough to cause severe poisoning.
Excretion
A few minutes after ingestion, strychnine is excreted unchanged in the urine, and accounts for about 5 to 15% of a sublethal dose given over 6 hours. Approximately 10 to 20% of the dose will be excreted unchanged in the urine in the first 24 hours. The percentage excreted decreases with the increasing dose. Of the amount excreted by the kidneys, about 70% is excreted in the first 6 hours, and almost 90% in the first 24 hours. Excretion is virtually complete in 48 to 72 hours.[51]
Treatment
There is no specific antidote for strychnine but recovery from exposure is possible with early supportive medical treatment. Strychnine poisoning demands aggressive management with early control of muscle spasms, intubation for loss of airway control, toxin removal (decontamination), intravenous hydration and potentially active cooling efforts in the context of hyperthermia as well as hemodialysis in kidney failure (to note, strychnine has not been shown to be removed by hemodialysis).[24] Strychnine poisoning in today's age generally results from herbal remedies and strychnine-containing rodenticides.[52] Moreover, management should be tailored to the patient's history of chief complaint and workup to rule out other causes. If a poisoned person is able to survive for 6 to 12 hours subsequent to initial dose, they have a good prognosis.[24] The patient should be kept in a quiet and darkened room, because excessive manipulation and loud noises may cause convulsions. Because these convulsions are extremely painful, appropriate analgesics should be administered. Treatment of strychnine poisoning involves oral administration of activated charcoal which adsorbs strychnine within the digestive tract; unabsorbed strychnine is removed from the stomach by gastric lavage, along with tannic acid or potassium permanganate solutions to oxidize strychnine. Activated charcoal may be beneficial, but its benefit remains unproven, to note its use should be avoided in any patient with a tenuous airway or altered mental status.[53] Seizures are controlled by anticonvulsants, such as phenobarbital or diazepam,[24] along with muscle relaxants such as dantrolene to combat muscle rigidity. Historically chloroform or heavy doses of chloral, bromide, urethane or amyl nitrite were used to restrain the convulsions. Because medications such as diazepam are not effective to relieve convulsions in all cases, concurrent use of barbiturates and/or propofol can be utilized.
The sine qua non of strychnine toxicity is the "awake" seizure, in which tonic-clonic activity occurs but the patient is alert and oriented throughout and afterwards.[54] Accordingly, George Harley (1829–1896) showed in 1850 that curare (wourali) was effective for the treatment of tetanus and strychnine poisoning. Curare causes paralysis, and it is important to note that, if seizure activity is present, the use of muscle paralysis will only mask the signs of ongoing seizure activity despite otherwise ongoing present brain damage.[55]
History
Strychnine was the first alkaloid to be identified in plants of the genus Strychnos, family Loganiaceae. Strychnos, named by Carl Linnaeus in 1753, is a genus of trees and climbing shrubs of the Gentianales order. The genus contains 196 various species and is distributed throughout the warm regions of Asia (58 species), America (64 species), and Africa (75 species). The seeds and bark of many plants in this genus contain strychnine.
The toxic and medicinal effects of Strychnos nux-vomica have been well known from the times of ancient India, although the chemical compound itself was not identified and characterized until the 19th century. The inhabitants of these countries had historical knowledge of the species Strychnos nux-vomica and Saint-Ignatius' bean (Strychnos ignatii). Strychnos nux-vomica is a tree native to the tropical forests on the Malabar Coast in Southern India, Sri Lanka and Indonesia, which attains a height of about 12 metres (39 ft). The tree has a crooked, short, thick trunk and the wood is close grained and very durable. The fruit has an orange color and is about the size of a large apple with a hard rind and contains five seeds, which are covered with a soft wool-like substance. The ripe seeds look like flattened disks, which are very hard. These seeds are the chief commercial source of strychnine and were first imported to and marketed in Europe as a poison to kill rodents and small predators. Strychnos ignatii is a woody climbing shrub of the Philippines. The fruit of the plant, known as Saint Ignatius' bean, contains as many as 25 seeds embedded in the pulp. The seeds contain more strychnine than other commercial alkaloids. The properties of S. nux-vomica and S. ignatii are substantially those of the alkaloid strychnine.
Strychnine was first discovered by French chemists Joseph Bienaimé Caventou and Pierre-Joseph Pelletier in 1818 in the Saint-Ignatius' bean.[56][57] In some Strychnos plants a 9,10-dimethoxy derivative of strychnine, the alkaloid brucine, is also present. Brucine is not as poisonous as strychnine. Historic records indicate that preparations containing strychnine (presumably) had been used to kill dogs, cats, and birds in Europe as far back as 1640.[51] It was also used during World War II by the Dirlewanger Brigade against civilian population.[58]
The structure of strychnine was first determined in 1946 by Sir Robert Robinson and in 1954 this alkaloid was synthesized in a laboratory by Robert B. Woodward. This is one of the most famous syntheses in the history of organic chemistry. Both chemists won the Nobel prize (Robinson in 1947 and Woodward in 1965).[51]
Strychnine has been used as a plot device in the author Agatha Christie's murder mysteries.[59]
Performance enhancer
Strychnine was popularly used as an athletic performance enhancer and recreational stimulant in the late 19th century and early 20th century, due to its convulsant effects. One notorious instance of its use was during the 1904 Olympics marathon, when track-and-field athlete Thomas Hicks was unwillingly administered a concoction of egg whites and brandy laced with a small amount of strychnine by his assistants to boost his stamina. Hicks won the race, but was hallucinating by the time he reached the finish line, and soon after collapsed.[60][61] Maximilian Theodor Buch proposed it as a cure for alcoholism around the same time. It was thought to be similar to coffee.[62][63] Its effects are well-described in H. G. Wells' novella The Invisible Man: the title character states "Strychnine is a grand tonic ... to take the flabbiness out of a man." Dr Kemp, an acquaintance replies: "It's the devil, ... It's the palaeolithic in a bottle."[64]
See also
- Strychnine poisoning
- Avicide
- "Poisoning Pigeons in the Park"
- The Mysterious Affair at Styles
- The Sonics "Strychnine"
References
- Retrieved from SciFinder. [May 7, 2018]
- "Strychnine". CDC – NIOSH Pocket Guide to Chemical Hazards.
- Everett AJ, Openshaw HT, Smith GF (1957). "The constitution of aspidospermine. Part III. Reactivity at the nitrogen atoms, and biogenetic considerations". Journal of the Chemical Society: 1120–1123. doi:10.1039/JR9570001120.
- "Strychnine". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Wells JC (2008). Longman Pronunciation Dictionary (3rd ed.). Longman. ISBN 978-1-4058-8118-0.
- Jones D (2011). Roach P, Setter J, Esling J (eds.). Cambridge English Pronouncing Dictionary (18th ed.). Cambridge University Press. ISBN 978-0-521-15255-6.
- Sharma RK (2008). Consice textbook of forensic medicine & toxicology. Elsevier.
- Munro, J. M. H. (1914-04-18). "Veronal Poisoning: Case of Recovery from 125 Grains". British Medical Journal. 1 (2781): 854–856. doi:10.1136/bmj.1.2781.854. ISSN 0007-1447. PMC 2300683. PMID 20767090.
An attempt was made to administer a soap-and-water enema, but the sphincter was not acting. After hypodermic injection of 1/45 grain [1.44 mg] strychnine, a second attempt was made, and a good evacuation of the bowel followed, after which half a pint [284 ml] of normal saline was injected and retained. [...] We decided to adhere to the treatment already commenced – namely, periodical rectal injection of saline and withdrawals of urine by catheter, with oxygen inhalation for cyanosis, and strychnine hypodermically as the pulse weakened.
- Bonjoch J, Solé D (September 2000). "Synthesis of Strychnine". Chemical Reviews. 100 (9): 3455–3482. doi:10.1021/cr9902547. PMID 11777429.
- Dewick PM (2009). Medicinal natural products: a biosynthetic approach (3rd ed.). Chichester: A John Wiley & Sons. pp. 377–378. ISBN 978-0-470-74167-2.
- Hong, Benke; Grzech, Dagny; Caputi, Lorenzo; Sonawane, Prashant; López, Carlos E. Rodríguez; Kamileen, Mohamed Omar; Hernández Lozada, Néstor J.; Grabe, Veit; O'Connor, Sarah E. (July 2022). "Biosynthesis of strychnine". Nature. 607 (7919): 617–622. Bibcode:2022Natur.607..617H. doi:10.1038/s41586-022-04950-4. PMC 9300463. PMID 35794473.
- Treimer JF, Zenk MH (November 1979). "Purification and properties of strictosidine synthase, the key enzyme in indole alkaloid formation". European Journal of Biochemistry. 101 (1): 225–233. doi:10.1111/j.1432-1033.1979.tb04235.x. PMID 510306.
- Heimberger SI, Scott AI (1973). "Biosynthesis of strychnine". Journal of the Chemical Society, Chemical Communications (6): 217–218. doi:10.1039/C39730000217.
- Tatsis EC, Carqueijeiro I, Dugé de Bernonville T, Franke J, Dang TT, Oudin A, et al. (August 2017). "A three enzyme system to generate the Strychnos alkaloid scaffold from a central biosynthetic intermediate". Nature Communications. 8 (1): 316. Bibcode:2017NatCo...8..316T. doi:10.1038/s41467-017-00154-x. PMC 5566405. PMID 28827772.
- Wieland H, Gumlich W (1932). "Über einige neue Reaktionen der Strychnos – Alkaloide. XI" [On some new reactions of the Strychnos alkaloids. XI]. Justus Liebig's Annalen der Chemie (in German). 494: 191–200. doi:10.1002/jlac.19324940116.
- Nicolaou KC, Vourloumis D, Winssinger N, Baran PS (January 2000). "The Art and Science of Total Synthesis at the Dawn of the Twenty-First Century". Angewandte Chemie. 39 (1): 44–122. doi:10.1002/(SICI)1521-3773(20000103)39:1<44::AID-ANIE44>3.0.CO;2-L. PMID 10649349.
- Robinson R (1952). "Molecular structure of Strychnine, Brucine and Vomicine". Progress in Organic Chemistry. 1: 2.
- Nicolaou KC, Sorensen EJ (1996). Classics in Total Synthesis: Targets, Strategies, Methods. Wiley. ISBN 978-3-527-29231-8.
- Woodward RB (1954). "The total synthesis of strychnine". Experientia. 76 (Suppl 2): 213–228. doi:10.1021/ja01647a088. PMID 13305562. S2CID 42677858.
- Woodward RB (1963). "The total synthesis of strychnine". Experientia. 19 (Suppl 2): 213–228. doi:10.1016/S0040-4020(01)98529-1. PMID 13305562.
- Waring RH, Steventon GB, Mitchell SC (2007). Molecules of death. Imperial College Press.
- Brams M, Pandya A, Kuzmin D, van Elk R, Krijnen L, Yakel JL, et al. (March 2011). "A structural and mutagenic blueprint for molecular recognition of strychnine and d-tubocurarine by different cys-loop receptors". PLOS Biology. 9 (3): e1001034. doi:10.1371/journal.pbio.1001034. PMC 3066128. PMID 21468359.
- "Strychnine". INCHEM: Chemical Safety Information from Intergovernmental Organizations.
- "CDC – The Emergency Response Safety and Health Database: Biotoxin: Strychnine – NIOSH". www.cdc.gov. Retrieved 2016-01-02.
- Tucker RK, Haegele MA (September 1971). "Comparative acute oral toxicity of pesticides to six species of birds". Toxicology and Applied Pharmacology. 20 (1): 57–65. doi:10.1016/0041-008X(71)90088-3. PMID 5110827.
- RTECS (1935)
- Moraillon R, Pinoult L (1978). "Diagnostic et traitement d'intoxications courantes des carnivores" [Diagnosis and treatment of common poisoning of carnivores]. Rec Med Vet (in French). 174 (1–2): 36–43.
- Longo VG, Silvestrini B, Bovet D (May 1959). "An investigation of convulsant properties of the 5-7-diphenyl-1-3-diazadamantan-6-01 (1757-I. S.)". The Journal of Pharmacology and Experimental Therapeutics. 126 (1): 41–49. PMID 13642285.
- Setnikar I, Murmann W, Magistretti MJ, Da Re P (February 1960). "Amino-methylchromones, brain stem stimulants and pentobarbital antagonists". The Journal of Pharmacology and Experimental Therapeutics. 128: 176–181. PMID 14445192.
- Haas H (October 1960). "[On 3-piperidino-1-phenyl-1-bicycloheptenyl-1-propanol (Akineton). 2]". Archives Internationales de Pharmacodynamie et de Therapie. 128: 204–238. PMID 13710192.
- Prasad CR, Patnaik GK, Gupta RC, Anand N, Dhawan BN (November 1981). "Central nervous system stimulant activity of n-(delta 3-chromene-3-carbonyl)-4 iminopyridine (compound 69/224)". Indian Journal of Experimental Biology. 19 (11): 1075–1076. PMID 7338366.
- Zapata-Ortiz V, Castro De La Mata R, Barantes-Campos R (July 1961). "[The anticonvulsive action of cocaine]" [The anticonvulsive action of cocaine]. Arzneimittel-Forschung (in German). 11: 657–662. PMID 13787891.
- Sandberg F, Kristianson K (September 1970). "A comparative study of the convulsant effects of strychnos alkaloids". Acta Pharmaceutica Suecica. 7 (4): 329–336. PMID 5480076.
- Spector WS (1956). Handbook of Toxicology. Vol. 1. Philadelphia: W. B. Saunders Company. p. 286.
- Ward JC, Crabtree DG (1942). "Strychnine X. Comparative accuracies of stomach tube and intraperitoneal injection methods of bioassay". Journal of the American Pharmaceutical Association. 31 (4): 113–115. doi:10.1002/jps.3030310406.
- Duverneuil C, de la Grandmaison GL, de Mazancourt P, Alvarez JC (April 2004). "Liquid chromatography/photodiode array detection for determination of strychnine in blood: a fatal case report". Forensic Science International. 141 (1): 17–21. doi:10.1016/j.forsciint.2003.12.010. PMID 15066709.
- Santhosh GJ, Joseph W, Thomas M (July 2003). "Strychnine poisoning". The Journal of the Association of Physicians of India. 51: 739–740. PMID 14621058.
- Zenz C, Dickerson OB, Horvath EP (1994). Occupational Medicine (3rd ed.). St Louis. p. 640.
- Palatnick W, Meatherall R, Sitar D, Tenenbein M (2008). "Toxicokinetics of acute strychnine poisoning". Journal of Toxicology. Clinical Toxicology. 35 (6): 617–620. doi:10.3109/15563659709001242. PMID 9365429.
- Lewis RG (1996). Sax's Dangerous Properties of Industrial Materials. Vol. 1–3 (9th ed.). New York: Van Nostrand Reinhold. p. 3025.
- Goodman LS, Gilman AG, Gilman AM (1985). The pharmalogical basis of therapeutics. New York Macmillan Publishing & Co., Inc.
- Gossel TA, Bricker JD (1994). Principles of Clinical Toxicology (3rd ed.). New York: Raven Press. p. 351.
- Migliaccio E, Celentano R, Viglietti A, Viglietti G (1990). "[Strychnine poisoning. A clinical case]". Minerva Anestesiologica. 56 (1–2): 41–42. PMID 2215981.
- Ellenhorn MJ, Schonwald S, Ordog G, Wasserberger J, eds. (1997). "Strychnine". Medical Toxicology: Diagnosis and Treatment of Human Poisoning. Baltimore: Williams & Wilkins. pp. 1660–1662.
- "Chemistry in its element – strychnine". Royal Society of Chemistry. Retrieved 18 May 2016.
- Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, et al. (February 2010). "The molecular receptive ranges of human TAS2R bitter taste receptors". Chemical Senses. 35 (2): 157–170. doi:10.1093/chemse/bjp092. PMID 20022913.
- Born S, Levit A, Niv MY, Meyerhof W, Behrens M (January 2013). "The human bitter taste receptor TAS2R10 is tailored to accommodate numerous diverse ligands". The Journal of Neuroscience. 33 (1): 201–213. doi:10.1523/JNEUROSCI.3248-12.2013. PMC 6618634. PMID 23283334.
- Meyerhof W, Born S, Brockhoff A, Behrens M (2011). "Molecular biology of mammalian bitter taste receptors. A review". Flavour and Fragrance Journal. 26 (4): 260–268. doi:10.1002/ffj.2041.
- Lambert JR, Byrick RJ, Hammeke MD (May 1981). "Management of acute strychnine poisoning". Canadian Medical Association Journal. 124 (10): 1268–1270. PMC 1705440. PMID 7237316.
- Gupta RC (2009). Handbook of toxicology of chemical warfare agents. Elsevier/Academic Press. ISBN 978-0-12-800159-2. OCLC 433545336.
- Gupta RC, Patocka J (2009). Handbook of Toxicology of Chemical Warfare Agents. London: Academic Press. p. 199. ISBN 9780080922737.
- Katz J, Prescott K, Woolf AD (September 1996). "Strychnine poisoning from a Cambodian traditional remedy". The American Journal of Emergency Medicine. 14 (5): 475–477. doi:10.1016/S0735-6757(96)90157-6. PMID 8765115.
- Smith BA (1990). "Strychnine poisoning". The Journal of Emergency Medicine. 8 (3): 321–325. doi:10.1016/0736-4679(90)90013-L. PMID 2197324.
- Boyd RE, Brennan PT, Deng JF, Rochester DF, Spyker DA (March 1983). "Strychnine poisoning. Recovery from profound lactic acidosis, hyperthermia, and rhabdomyolysis". The American Journal of Medicine. 74 (3): 507–512. doi:10.1016/0002-9343(83)90999-3. PMID 6829597.
- "Rapid Sequence Termination (RST) of status epilepticus". 2014-06-04.
- Pelletier PP, Caventou JB (1818). "Note sur un nouvel alkalai" [Note on a new alkali]. Annales de Chimie et de Physique (in French). 8: 323–324.
- Pelletier PP, Caventou JB (1819). "Mémoire sur un nouvel alcali vegetal (la strychnine) trouvé dans la feve de Saint-Ignace, la noix vomique, etc" [Memoir on a new vegetable alkali (strychnine) found in the St. Ignatius bean, the nux-vomica, etc)]. Annales de Chimie et de Physique (in French). 10: 142–176.
- Grunberger R (1971). The 12-Year Reich: A Social History of Nazi Germany, 1933–1945. Holt, Rinehart and Winston. p. 104.
- "Killed by Agatha Christie: Strychnine and the detective novel". www.open.edu. Open university. Retrieved 27 July 2017.
- "The Deafening Roar of the Shrug". The New York Times. July 29, 2007.
- "Thomas Hicks". Olympedia. Retrieved January 17, 2021.
- Inglis-Arkell E (11 June 2013). "Rat poison strychnine was an early performance-enhancing drug". io9. Gawker Media. Retrieved 23 Nov 2015.
- "Strictly strychnine – medicines to be avoided by athletes".
- Wells HG. The Invisible Man.
