VX (nerve agent)
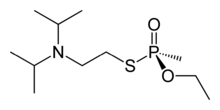 SP-(−)-VX enantiomer | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
S-{2-[Di(propan-2-yl)amino]ethyl} O-ethyl methylphosphonothioate | |
| Other names
[2-(Diisopropylamino)ethyl]-O-ethyl methylphosphonothioate Ethyl {[2-(diisopropylamino)ethyl]sulfanyl}(methyl)phosphinate Ethyl N-2-diisopropylaminoethyl methylphosphonothiolate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| MeSH | VX |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H26NO2PS | |
| Molar mass | 267.37 g·mol−1 |
| Density | 1.0083 g cm−3 |
| Melting point | −51 °C (−60 °F; 222 K) |
| Boiling point | 300 °C (572 °F; 573 K) |
| log P | 2.047 |
| Vapor pressure | 0.09 Pa |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 159 °C (318 °F; 432 K)[3] |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
7 μg/kg (intravenous, rat)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
| Part of a series on | |||
| Chemical agents | |||
|---|---|---|---|
| Lethal agents | |||
| Incapacitating agents | |||
|
|||
| |||
VX is an extremely toxic synthetic chemical compound in the organophosphorus class, specifically, a thiophosphonate. In the class of nerve agents, it was developed for military use in chemical warfare after translation of earlier discoveries of organophosphate toxicity in pesticide research. In recent years, VX was found to be the agent used in the assassination of Kim Jong-nam. In its pure form, VX is an oily, relatively non-volatile, liquid that is amber-like in colour.[4] Because of its low volatility, VX persists in environments where it is dispersed.[5]
VX, short for "venomous agent X",[6] is one of the best known of the V nerve agents and was first discovered at Porton Down by Ranajit Ghosh in England during the early 1950s based on research first done by Gerhard Schrader, a chemist working for IG Farben in Germany during the 1930s. It is now one of a broader V-series of agents which are classified as nerve agents and have been used as a chemical weapon in various recorded deadly attacks. VX fatalities occur with exposure to tens of milligram quantities via inhalation or absorption through skin; VX is thus more potent than sarin, another nerve agent with a similar mechanism of action. On such exposure, these agents severely disrupt the body's signaling between the nervous and muscular systems, leading to a prolonged neuromuscular blockade, flaccid paralysis of all the muscles in the body including the diaphragm, and death by asphyxiation.[7]
The danger of VX, in particular, lies in direct exposure to the chemical agent persisting where it was dispersed, and not through its evaporating and being distributed as a vapor; it is not considered a vapor hazard due to its relative non-volatility. VX is considered an area denial weapon due to these physical and biochemical characteristics.[8] As a chemical weapon, it is categorized as a weapon of mass destruction by the United Nations and is banned by the Chemical Weapons Convention of 1993,[9] where production and stockpiling of VX exceeding 100 grams (3.53 oz) per year is outlawed. The only exception is for "research, medical or pharmaceutical purposes outside a single small-scale facility in aggregate quantities not exceeding 10 kg (22 lb) per year per facility".[10]
Physical properties
VX is an odorless and tasteless[11][12] chiral organophosphorous chemical with a molecular weight of 267.37 g/mol.[13] Under standard conditions it is an amber-coloured liquid with a boiling point of 298 °C (568 °F), and a freezing point of −51 °C (−60 °F).[14] Its density is similar to that of water.[15] It has a log P value of 2.047, meaning it is relatively hydrophobic with about 100-fold more partitioning into octanol, over water.[16] Its low vapor pressure of 0.09 pascals (1.3×10−5 psi) gives it a low volatility, resulting in a high persistence in the environment.[17]
When weaponized, it can be dispersed as a liquid, aerosol or as a mixture with a clay or talc thickening agent.[17]
Mechanism of action
VX is an acetylcholinesterase inhibitor.[18] It blocks the function of the enzyme acetylcholinesterase (AChE). Normally, when a motor neuron is stimulated, it releases the neurotransmitter acetylcholine (ACh) into the space between the neuron and an adjacent muscle cell, the synaptic cleft. When acetylcholine binds to nicotinic receptors at the neuromuscular junction, it stimulates muscle contraction. To avoid a state of constant muscle contraction, the acetylcholine is then broken down (hydrolysed) into the inactive substances acetic acid and choline by AChE. VX blocks the action of AChE, resulting in an accumulation of acetylcholine in the space between the neuron and muscle cell. On a molecular level, this leads to the ongoing stimulation and eventual fatigue of all affected muscarinic and nicotinic ACh receptors. This results in initial violent contractions, followed by sustained supercontraction restricted to the fluid (sarcoplasm) of the subjunctional endplate and prolonged, depolarizing neuromuscular blockade.[19]The prolonged blockade results in flaccid paralysis of all the muscles in the body, and it is such sustained paralysis of the diaphragm muscle that causes death by asphyxiation.[7] Accumulation of acetylcholine in the brain also causes neuronal excitotoxicity, due to activation of nicotinic receptors and glutamate release.[20]
The extreme toxicity of VX is partly due to the fact that the inhibitor was designed to be an excellent structural mimic for the transition state of the natural substrate (acetylcholine) of acetylcholinesterase. VX has a very high "on-rate" to react with the target enzyme and form a stable P-O-C bond (phosphorylation).[21] However, compared with other highly toxic nerve agents like soman or sarin, VX undergoes relatively slow "aging". Aging is a time-dependent side reaction (loss of an alkoxyl group) that occurs on nerve agents after phosphonylation and renders the nerve agent-acetylcholinesterase complex highly resistant to regeneration by any known antidote. Slower aging by VX suggests it should be possible to develop more effective antidotes and treatments.[22]
The reaction products of acetylcholinesterase with VX before and after the "aging" reaction were solved near atomic resolution by X-ray crystallography to aid in antidote development.[23][24] The X-ray structures revealed the specific parts of the VX molecule that interact with key residues and sub-sites of the target enzyme. The structural kinetic of phosphonylation followed by aging also showed an unexpected conformational change in the catalytic triad suggestive of an "induced fit" between the VX molecule and acetylcholinesterase.
Chemistry
Synthesis
VX is chiral at its phosphorus atom. The individual enantiomers are identified as SP-(−)-VX, and RP-(+)-VX (where the "P" subscript highlights that the chirality is at phosphorus).[1]
VX is produced via the transester process, which gives a racemic mixture of the two enantiomers. This entails a series of steps whereby phosphorus trichloride is methylated to produce methyl phosphonous dichloride. The resulting material is reacted with ethanol to form a diester. This is then transesterified with N,N-diisopropylaminoethanol to produce the mixed phosphonite. Finally, this immediate precursor is reacted with sulfur to form VX.
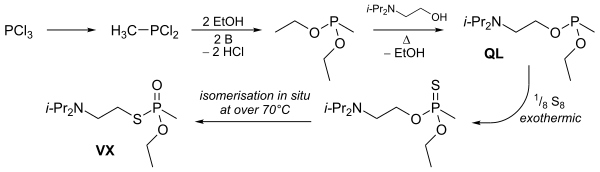
VX can also be delivered in binary chemical weapons which mix in-flight to form the agent prior to release. Binary VX is referred to as VX2,[25] and is created by mixing O-(2-diisopropylaminoethyl) O′-ethyl methylphosphonite (Agent QL) with elemental sulfur (Agent NE) as is done in the Bigeye aerial chemical bomb. It may also be produced by mixing with sulfur compounds, as with the liquid dimethyl polysulfide mixture (Agent NM) in the canceled XM736 8-inch projectile program.[26]
Solvolysis
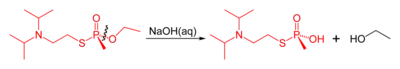
Like other organophosphorus nerve agents, VX may be destroyed by reaction with strong nucleophiles. The reaction of VX with concentrated aqueous sodium hydroxide results in two competing solvolysis reactions: cleavage of either the P–O or P–S esters. Although the P–S cleavage is the dominant pathway, the product of P–O bond cleavage is the toxic phosphonic thioester EA-2192 and both reactions are slow.[27] In contrast, reaction with the hydroperoxide anion (hydroperoxidolysis) leads to exclusive cleavage of the P–S bond and a more rapid overall reaction.[27][28]

Medical aspects
Symptoms of exposure
Early symptoms of percutaneous exposure (skin contact) include local sweating and muscular twitching at the area of exposure, followed by nausea or vomiting. Early symptoms of exposure to VX vapor include rhinorrhea (runny nose) and/or tightness in the chest with shortness of breath (bronchial constriction). Miosis (pinpointing of the pupils) may be an early sign of agent exposure, but is not usually used as the only indicator of exposure.[29]
Toxicology
VX is a "particularly toxic nerve agent". The potentially fatal dose is only slightly higher than the dose having any effect at all, and the effects of a fatal dose are so rapid that there is little time for treatment.[5] The median lethal dose (LD50), the exposure required to kill half of a tested population, as estimated for 70 kg human males via exposure to the skin is reported to be 5–10 mg (0.00035 oz), and the lethal concentration time (LCt50), measuring the concentration of the vapor or aerosol per length of time exposed, is estimated for VX to be 10–15 mg·min/m3 for exposure time of two minutes at a minute volume of 15 L (minute volume of 15 L corresponds to slight physical activity like slow walking).[30][31][5]
Treatment
When treating VX exposure, primary consideration is given to removal of the liquid agent from the skin, before removal of the individual to an uncontaminated area or atmosphere. After this, the victim is decontaminated by washing the contaminated areas with household bleach and flushing with clean water, followed by removal of contaminated clothing and further skin decontamination. When possible, decontamination is completed before the casualty is taken for further medical treatment.[32][33][34]
An individual known to have been exposed to a nerve-agent, or who exhibits definite signs or symptoms of nerve-agent exposure is generally given the antidotes atropine and pralidoxime (2-PAM), and in the case of convulsions an injected sedative/antiepileptic such as diazepam.[35] In several nations the nerve agent antidotes are issued for military personnel in the form of an autoinjector such as the United States military Mark I NAAK.[29]
Atropine blocks a subset of acetylcholine receptors known as muscarinic acetylcholine receptors (mAchRs), so that the buildup of acetylcholine produced by loss of the acetylcholinesterase function has a reduced effect on their target receptor. 2-PAM reactivates the acetylcholinesterase enzyme (AChE), thus reversing the effects of VX. VX and other organophosphates block AChE activity by binding to and covalently inactivating the enzyme via transfer of the phosphonate moiety from VX to the active site of AChE; this inactivates AChE and produces an inactive by-product from the remaining portion of the VX molecule. Pralidoxime (2-PAM) removes this phosphate group.
Diagnostic tests
Controlled studies in humans have shown that minimally toxic doses cause 70–75% depression of erythrocyte cholinesterase within several hours of exposure. The serum level of ethyl methylphosphonic acid (EMPA), a VX hydrolysis product, was measured to confirm exposure in one poisoning victim. There also exist procedures for determination of VX hydrolysis products in urine and of VX adducts to albumin in blood.[36]
History
Discovery
The chemists Ranajit Ghosh and J. F. Newman discovered the V-series nerve agents at the British firm ICI in 1952, patenting diethyl S-2-diethylaminoethyl phosphonothioate (agent VG) in November 1952. Further commercial research on similar compounds ceased in 1955 when its lethality to humans was discovered. The U.S. started production of large amounts of VX in 1961 at Newport Chemical Depot.
The discovery occurred when the chemists were investigating a class of organophosphate compounds (organophosphate esters of substituted aminoethanethiols).[37] Like Gerhard Schrader, an earlier investigator of organophosphates, Ghosh found that they were quite effective pesticides. In 1954, ICI put one of them on the market under the trade name Amiton. It was subsequently withdrawn, as it was too toxic for safe use. The toxicity did not go unnoticed, and samples of it were sent to the British Armed Forces research facility at Porton Down for evaluation. After the evaluation was complete, several members of this class of compounds became a new group of nerve agents, the V agents. The best-known of these is probably VX, assigned the UK Rainbow Code Purple Possum, with the Russian V-Agent (VR) coming a close second (Amiton is largely forgotten as VG). The name is a contraction of the words "venomous agent X".[38]
Beginning in 1959, the United States Army began volunteer testing of VX in humans. Dr. Van M. Sim underwent an intravenous infusion of VX to evaluate its effects and to establish a baseline for future experimentation. After approximately 3.5 hours following initial administration of the agent, Sim suddenly became pale and delirious. The experiment was immediately terminated to preserve his life. In their conclusion, the researchers estimated that 2.12 μg/kg of VX delivered intravenously over the course of several hours would be the maximum tolerable dosage and that any more would risk death in a human subject.[39]
Use as a weapon
In 1988, a United Nations inquiry established that Cuba was responsible for deploying VX against Angolan insurgents during the Angolan Civil War.[40][41] UN toxicologists obtained trace elements of VX from soil, water, and plant samples taken from areas where Cuban troops had recently carried out counter-insurgency operations.[40] Patients demonstrating symptoms of exposure to nerve agents first began appearing in Angolan hospitals around 1984.[42]
There was evidence of a combination of chemical agents having been used by Iraq against the Kurds in the Halabja chemical attack in 1988 under Saddam Hussein, including VX.[43] Hussein later testified to UNSCOM that Iraq had researched VX, but had failed to weaponize the agent due to production failure. After U.S. and allied forces invaded Iraq, no VX agent or production facilities were found. However, UNSCOM laboratories detected traces of VX on warhead remnants.[44][45]
In December 1994 and January 1995, Masami Tsuchiya of Aum Shinrikyo synthesized 100 to 200 grams (3.5 to 7.1 oz) of VX which was used to attack three people. Two people were injured and one 28-year-old man died, who was the first victim of VX ever documented in the world at that time. The VX victim, whom Shoko Asahara had suspected as a spy, was attacked at 7:00 am on 12 December 1994 on the street in Osaka by Tomomitsu Niimi and another AUM member, who sprinkled the nerve agent on his neck. He chased them for about 90 metres (100 yd) before collapsing, dying ten days later without ever coming out of a deep coma. Doctors in the hospital suspected at the time he had been poisoned with an organophosphate pesticide, but the cause of death was pinned down only after cult members arrested for the Tokyo subway sarin attack confessed to the killing. Metabolites of VX such as ethyl methylphosphonate, methylphosphonic acid and diisopropyl-2-(methylthio)ethylamine were later found in samples of the victim's blood seven months after his murder.[46] Unlike the cases for sarin gas (the Matsumoto incident and the attack on the Tokyo subway), VX was not used for mass murder.
On 13 February 2017, Kim Jong-nam, half-brother of North Korean leader Kim Jong-un, died after an assault at Kuala Lumpur International Airport in Malaysia. According to the authorities he was murdered by poisoning with VX which was found on his face.[47][48] The authorities further reported that one of the women suspected of applying the nerve agent experienced some physical symptoms of VX-poisoning.[49] The director of a non-proliferation research program of the Middlebury Institute of International Studies at Monterey stated that VX fumes would have killed the suspected attackers even if they had been wearing gloves, suggesting that the VX was applied as two non-lethal components that would mix to form VX only on the victim's face.[50]
Worldwide stockpiles
Some countries known to possess VX are the United States, the United Kingdom, Russia,[51] North Korea,[52] and Syria.[53] A Sudanese pharmaceutical facility, the Al-Shifa pharmaceutical factory, was bombed by the U.S. in 1998 acting on information that it produced VX and that the origin of the agent was associated with both Iraq and Al Qaeda.[44] The U.S. had obtained soil samples identified as containing O-ethyl hydrogen methylphosphonothioate (EMPTA), a chemical used in the production of VX which may also have commercial applications. Chemical weapons experts later suggested that the widely used fonofos organophosphate insecticide could have been mistaken for EMPTA.[54] Cuba obtained VX during the 1980s and deployed it during its military intervention in Angola.[40]
In 1969, the U.S. government cancelled its chemical weapons programs, banned the production of VX in the United States, and began the destruction of its stockpiles of agents by a variety of methods. Early disposal included the U.S. Army's CHASE (Cut Holes And Sink 'Em) program, in which old ships were filled with chemical weapons stockpiles and then scuttled. CHASE 8 was conducted on 15 June 1967, in which the steamship Cpl. Eric G. Gibson was filled with 7,380 VX rockets and scuttled in 2,200 m (7,200 ft) of water off the coast of Atlantic City, New Jersey. Incineration was used for VX stockpile destruction starting in 1990 with Johnston Atoll Chemical Agent Disposal System in the North Pacific with other incineration plants following at Deseret Chemical Depot, Pine Bluff Arsenal, Umatilla Chemical Depot and Anniston Army Depot with the last of the VX inventory destroyed on 24 December 2008.[55]
Stockpile elimination
Worldwide, VX disposal has continued since 1997 under the mandate of the Chemical Weapons Convention. When the convention entered force, the parties declared worldwide stockpiles of 19,586 tonnes (21,590 short tons) of VX. As of December 2015, 98% of the stockpiles had been destroyed.[56]
In fiscal year 2008, the U.S. Department of Defense released a study finding that the United States had dumped at least 112 tonnes (124 short tons) of VX into the Atlantic Ocean off the coasts of New York/New Jersey and Florida between 1969 and 1970. This material consisted of nearly 22,000 M55 rockets, 19 bulk containers holding 640 kg (1,400 lb) each, and one M23 chemical landmine.[57]
The Newport Chemical Depot began VX stockpile elimination using chemical neutralization in 2005. VX was hydrolyzed to much less toxic byproducts by using concentrated caustic solution, and the resulting waste was then shipped off-site for further processing. Technical and political issues regarding this secondary byproduct resulted in delays, but the depot completed their VX stockpile destruction in August 2008.[58]
The remaining VX stockpile in the U.S. was treated by the Blue Grass Chemical Agent-Destruction Pilot Plant in Kentucky, part of the Program Executive Office, Assembled Chemical Weapons Alternatives program. The program was established as an alternative to the incineration process successfully used by the Army Chemical Materials Agency, which completed its stockpile destruction activities in March 2012. The Blue Grass Pilot Plant has been plagued by repeated cost over-runs and schedule slippages since its inception.[59]
In Russia, the U.S. provided support for these destruction activities with the Nunn-Lugar Global Cooperation Initiative.[60] The Initiative has been able to convert a former chemical weapons depot at Shchuchye, Kurgan Oblast into a facility to destroy those chemical weapons. The new facility, which opened in May 2009, has been working on eliminating the nearly 5,400 tonnes (5,950 short tons) of nerve agents held at the former storage complex. However, this facility only held about 14% of Russian chemical weapons, which were stored at seven sites.[61]
In popular culture
One of the best-known references to VX in popular culture is its use in the 1996 film The Rock,[62][63] which centers on a threatened VX attack on San Francisco from the island of Alcatraz. The film uses artistic license, notably with VX being ascribed corrosive powers it does not possess, permitting an early scene in which a VX victim is shown with his face melting, rather than dying through asphyxiation. It is also shown as being bright green, a colour often used in Hollywood to represent 'danger' when applied to substances, rather than amber. As well, its delivery and dispersal mechanism is based around a chain of fictional glass balls that hold the substance as a gel solution.
Other references to VX are found in the 2012 film It's a Disaster in which it is revealed that a nearby dirty bomb attack was a VX attack, prompting the four couples to contemplate a suicide pact, as well as the 2015 film Mission: Impossible – Rogue Nation, in which series protagonist Ethan Hunt steals VX nerve gas from Chechen separatists on their way to Syria. Also season 5 of the TV series 24, has a similar storyline.[64] The fifth episode of the 2020 anime series The Millionaire Detective Balance: Unlimited features a tear gas bomb with canisters loaded with VX gas and placed inside a cabinet of a safe room within the embassy; the protagonist Daisuke Kambe and two other characters were trapped inside the room after relocating themselves due to security reasons, and figuring out how to escape before the bomb detonates.
The album VIVIsectVI by the industrial band Skinny Puppy contains a song about chemical weapons called "VX Gas Attack".
In the BBC show Spooks, series 2 episode 5, a dirty bomb using VX is said to have gone off in a 'training exercise'.
In the video game Everybody's Gone to the Rapture, VX is alluded to as a nerve agent used by the government to contain a pattern which infects and kills humans and other animals.
In the book Nightshade, the twelfth book in the Alex Rider Series, VX plays a major role, as it is used by terrorists in an attempt kill over half of the British government.
The second episode of the TV series Seal Team (season 1) focuses on a chemical weapons lab in an abandoned hospital, producing VX gas.
In the Netflix show Designated Survivor, agent Hannah Wells is killed by VX in season 3, episode 7.
In the CBS show MacGyver, season 2 episode 9, a VX canister is the main plot point.
In the Crackle show Startup, American soldiers discover a computer used by apparent terrorists in Aleppo, Syria. "VX components" are displayed as an item for purchase on the computer, which is logged into Araknet, a dark web created by the protagonists of the series.
See also
- Dugway sheep incident
- EA-1763
- List of Rainbow Codes
- Novichok agent
References
- John H, Balszuweit F, Kehe K, Worek F & Thiermann H (2015). "Toxicokinetic Aspects of Nerve Agents and Vesicants". In Gupta, Ramesh C. (ed.). Handbook of Toxicology of Chemical Warfare Agents (2nd ed.). Cambridge, MA: Academic Press. pp. 817–856, esp. 823 [Fig.56.1]. ISBN 978-0128004944. Retrieved March 22, 2017.
{{cite book}}: CS1 maint: multiple names: authors list (link) - Chambers, Michael. "Substance Name: VX". ChemIDplus. U.S. National Library of Medicine, National Institutes of Health. Retrieved 24 February 2017.
- "Material Safety Data Sheet: Nerve Agent (VX)". ilpi.com. Interactive Learning Paradigms Incorporated. December 22, 2000 [1998]. Retrieved October 25, 2007.
- "CDC | Facts About VX". emergency.cdc.gov. Centers for Disease Control and Prevention. Archived from the original on 2018-03-07. Retrieved 2018-03-20.
- FAS Staff (2013). "Types of Chemical Weapons: Nerve Agents [Table. Toxicological Data]". Washington, D.C.: Federation of American Scientists [FAS]. Archived from the original on November 26, 2016. Retrieved March 22, 2017.
- Bethany Halford (September 26, 2016). "Detoxifying VX". Chemical & Engineering News. 94 (38): 10–11. doi:10.1021/cen-09438-scicon001.
- Sidell, Frederick R. (1997). "Chapter 5: Nerve Agents" (PDF). Medical Aspects of Chemical and Biological Warfare. p. 142ff.
- Takafuji, Ernest T.; Kok, Allart B. (1997). "Chapter 4: The Chemical Warfare Threat and the Military Healthcare Provider" (PDF). Medical Aspects of Chemical and Biological Warfare. p. 123.
Given favorable weather conditions, the use of persistent agents such as mustard and VX may pose a threat for many days. Such agents can deny or interfere with enemy occupation of terrain or use of equipment
- "Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on their Destruction. Annex on Chemicals". United Nations Organisation for the Prohibition of Chemical Weapons.
- OPCW (2005). "Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on their Destruction" (PDF). OPCW.org. Organization for the Prohibition of Chemical Weapons (OPCW). p. 122. Retrieved 26 August 2016.
- "CDC | Facts About VX". emergency.cdc.gov. 2019-05-16. Retrieved 2022-04-27.
- Doyle, Gerry (2017-02-24). "What Is VX Nerve Agent? A Deadly Weapon, Rarely Seen". The New York Times. ISSN 0362-4331. Retrieved 2022-04-27.
- PubChem. "O-Ethyl S-(2-diisopropylaminoethyl) methylphosphonothioate". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-04-27.
- "Tx 60 | C11H26NO2PS". PubChem. Retrieved 2017-04-13.
- "Tx 60 | C11H26NO2PS". PubChem. Retrieved 2017-04-13.
- "Tx 60 | C11H26NO2PS". PubChem. Retrieved 2017-04-13.
- Croddy, Eric (October 1, 2002). "Dusty Agents and the Iraqi Chemical Weapons Arsenal". Nuclear Threat Initiative [NTI]. Washington, DC and Monterey, California: Middlebury Institute of International Studies, James Martin Center for Nonproliferation Studies. Retrieved March 22, 2017 – via NTI.org.
- McDowall, Jennifer (November 2005). "Acetylcholine Recepetors". European Molecular Biology Laboratory/European Bioinformatics Institute.
- Rash, John; Elmund, Julie (July 7, 1988). "Pathophsyiology of Anticholinesterase Agents" (PDF). Colorado State University Department of Anatomy and Neurobiology. Archived (PDF) from the original on April 12, 2022.
- Chen Y (2012). "Organophosphate-induced brain damage: mechanisms, neuropsychiatric and neurological consequences, and potential therapeutic strategies". Neurotoxicology. 33 (3): 391–400. doi:10.1016/j.neuro.2012.03.011. PMID 22498093.
- Ordentlich, Arie; Barak, Dov; Sod-Moriah, Gali; Kaplan, Dana; Mizrahi, Dana; Segall, Yoffi; Kronman, Chanoch; Karton, Yishai; Lazar, Arie; Marcus, Dino; Velan, Baruch; Shafferman, Avigdor (2004). "Stereoselectivity toward VX is Determined by Interactions with Residues of the Acyl Pocket as Well as of the Peripheral Anionic Site of AChE†". Biochemistry. 43 (35): 11255–65. doi:10.1021/bi0490946. PMID 15366935.
- Worek, F; Aurbek, N; Thiermann, H (2007). "Reactivation of organophosphate-inhibited human AChE by combinations of obidoxime and HI 6in vitro". Journal of Applied Toxicology. 27 (6): 582–8. doi:10.1002/jat.1241. PMID 17370251. S2CID 23783538.
- Millard, Charles B; Koellner, Gertraud; Ordentlich, Arie; Shafferman, Avigdor; Silman, Israel; Sussman, Joel L (1999). "Reaction Products of Acetylcholinesterase and VX Reveal a Mobile Histidine in the Catalytic Triad". Journal of the American Chemical Society. 121 (42): 9883–4. doi:10.1021/ja992704i.
- Wandhammer, Marielle; Carletti, Eugénie; Van Der Schans, Marcel; Gillon, Emilie; Nicolet, Yvain; Masson, Patrick; Goeldner, Maurice; Noort, Daan; Nachon, Florian (2011). "Structural Study of the Complex Stereoselectivity of Human Butyrylcholinesterase for the Neurotoxic V-agents". Journal of Biological Chemistry. 286 (19): 16783–9. doi:10.1074/jbc.M110.209569. PMC 3089521. PMID 21454498.
- Ellison, D. Hank (2007). Handbook of Chemical and Biological Agents. New York: CRC Press. p. 47. ISBN 978-0-8493-1434-6. Retrieved 2014-02-21.
- Adams, Robert W (1984-04-06). "Chemical Warfare in Future Military Operations". www.globalsecurity.org. Command and Staff College, United States Navy. Archived from the original on 2017-02-27. Retrieved 2018-03-20.
- Yang, Yu-Chu (1999). "Chemical Detoxification of Nerve Agent VX". Acc. Chem. Res. 32 (2): 109–15. doi:10.1021/ar970154s.
- Daniel, Kelly; Kopff, Laura A.; Patterson, Eric V.; et al. (2008). "Computational studies on the solvolysis of the chemical warfare agent VX". J. Phys. Org. Chem. 21 (4): 321–28. doi:10.1002/poc.1333.
- "US Army Toxic Chemical Agent Safety Standards" (PDF). DA PAM 385-61. Section 7-8 Self/Buddy Aid Procedures. US Army. Archived from the original (PDF) on December 24, 2003. Retrieved December 15, 2007.
- Lukey, Brian J.; Romano, James A. Jr.; Salem, Harry (2019-04-11). Chemical Warfare Agents: Biomedical and Psychological Effects, Medical Countermeasures, and Emergency Response. CRC Press. ISBN 9780429632969.
- Toxicology, National Research Council (US) Committee on (1997). Review of Acute Human-Toxicity Estimates for VX. National Academies Press (US).
- "CDC | Facts About VX". emergency.cdc.gov. 2019-05-16. Retrieved 2019-12-22.
- "ATSDR - Medical Management Guidelines (MMGs): Nerve Agents (GA, GB, GD, VX)". www.atsdr.cdc.gov. Retrieved 2019-12-22.
- "Facts About Nerve Agents". www.health.ny.gov. Retrieved 2019-12-22.
- "VX Recognition and Treatment" (PDF). Physicians for Human Rights. Retrieved December 14, 2019.
- R. Baselt (2017). Disposition of Toxic Drugs and Chemicals in Man (11th ed.). Seal Beach, CA: Biomedical Publications. pp. 2264–65.
- Ghosh, R.; Newman, J.E. (Jan 29, 1955). "A new group of organophosphorus pesticides". Chemistry and Industry: 118.
- Usborne, Tim (2016-06-28), Inside Porton Down: Britain's Secret Weapons Research Facility, Michael Mosley, Jonathan Lyle, Rob Evans, retrieved 2018-03-20
- Kazuo K. Kimura, Bernard P. McNamara, Van M. Sim (1960-07-01). "Intravenous Administration of VX in Man". Archived from the original on March 26, 2017. Retrieved 2017-03-25.
{{cite web}}: CS1 maint: multiple names: authors list (link) - Hawk, Kathleen Dupes; Villella, Ron; Varona, Adolfo Leyva de (July 30, 2014). Florida and the Mariel Boatlift of 1980: The First Twenty Days. University of Alabama Press. p. 250. ISBN 978-0-8173-1837-6. Retrieved October 11, 2014.
- Cuba's Pursuit of Biological Weapons: Fact or Fiction? Hearing Before the Subcommittee on Western Hemisphere, Peace Corps, and Narcotics Affairs of the Committee of Foreign Relations, United States Senate, One hundredth and seventh Congress, Second Session, Jun5 5, 2002 (PDF) (Report) (First ed.). Washington D.C.: Government Printing Office. 2002. p. 22. Retrieved 28 March 2018.
Already in 1988, the United Nations Security Council has been informed of use of toxic weapons by Soviet-supported Cuba in Angola. Belgian toxicologists had certified that residue of chemical weapons—including sarin and VX gas—had been found in plants, water and soil where Cuban troops were alleged to have used chemicals against Savimbi's troops.
- "Cubans using poison gas in Angola". The Lewiston Journal. Lewiston–Auburn, Maine. August 26, 1988. Retrieved July 28, 2015.
- BBC (March 16, 1988). "1988: Thousands die in Halabja gas attack". Retrieved March 1, 2012.
- John Pike. "Iraq Survey Group Final Report". Globalsecurity.org. Retrieved March 1, 2012.
- CIA (May 2, 2007). "Intelligence Update: Chemical Warfare Agent Issues Chemical Warfare Issues During the Persian Gulf War". Archived from the original on June 13, 2007. Retrieved Oct 22, 2012.
- Pamela Zurer. "Japanese cult used VX to slay member". Chemical and Engineering News. 1998, Vol 76 (no. 35), 7.
- Paddock, Richard C.; Sang-hun, Choe (2017-02-23). "Kim Jong-nam Was Killed by VX Nerve Agent, Malaysians Say". The New York Times. ISSN 0362-4331. Retrieved 2017-02-24.
- "Kim Jong-nam killing: VX nerve agent 'found on his face'". BBC News. 24 February 2017. Retrieved 2017-02-24.
- One suspect in Kim Jong Nam murder suffered effects of VX agent. The Star. 2017-2-24. Retrieved 23 February 2017.
- McCurry, Justin (2017-02-20). "What is the VX nerve agent that killed North Korean Kim Jong-nam?". The Guardian. Retrieved 2017-02-25.
- "VX". Council on Foreign Relations. Archived from the original on January 31, 2009. Retrieved June 12, 2007.
- Onyanga-Omara, Jane. "U.S. says North Korea used nerve agent VX to assassinate Kim Jong Un's half-brother". USA TODAY.
- "Synthèse nationale de renseignement déclassifié" [National synthesis of declassified intelligence] (PDF) (in French). 2013-08-21. Retrieved 2017-12-01.
- Claudine McCarthy (2005). "EMPTA (O-Ethyl methylphosphonothioic acid)" (Google Books excerpt). In Eric Croddy; James J. Wirtz (eds.). Weapons of mass destruction: an encyclopedia of worldwide policy, technology, and history. pp. 123–24. ISBN 1-85109-490-3. Retrieved 2014-02-21.
- "VX Destruction Milestone". U.S. Army Chemical Materials Agency. March 20, 2009. Archived from the original on 2009-03-27.
- "Annex 3". Report of the OPCW on the Implementation of the Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on Their Destruction in 2015 (Report). Organisation for the Prohibition of Chemical Weapons. 30 November 2016. p. 42. Retrieved 8 March 2017.
- "App_Q_Sea_Disposal_final" (PDF). denix.osd.mil. Retrieved September 7, 2009.
- "Depot Confirms VX Stockpile Eliminated". U.S. Army Chemical Materials Agency. Retrieved January 7, 2013.
- Schneidmiller, Chris (April 18, 2001). "U.S. Chemical Weapons Disposal Slippage "No Surprise," Expert Says". Nuclear Threat Initiative. Retrieved Oct 11, 2012.
- "Nunn-Lugar Global Cooperation Initiative". Defense Threat Reduction Agency and USSTRATCOM Center for Combating WMD. Retrieved 23 May 2012.
- Levy, Clifford J. (May 27, 2009). "In Siberia, the Death Knell of a Complex Holding a Deadly Stockpile". The New York Times. Retrieved April 9, 2010.
- "Molecular dynamics to combat chemical terrorism". Chemistry World. Royal Society of Chemistry. 31 January 2012.
- Zion, Ilan Ben (29 August 2013). "Vital sarin antidote missing from gas mask kits". Times of Israel.
- 24 - Day 5: 12:00 p.m.-1:00 p.m., 30 January 2006.
External links
 Media related to VX nerve agent at Wikimedia Commons
Media related to VX nerve agent at Wikimedia Commons- Oxford website on Nerve Agents
- Questions and Answers for VX
- CDC Facts About VX
- U.S. Army's Chemical Materials Agency (CMA)
- CBW Info
- Decommissioning Surplus VX – Article from The New York Times
