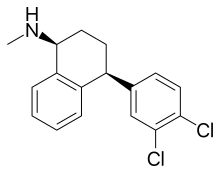
Sertraline
Sertraline, sold under the brand name Zoloft among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class.[9] The efficacy of sertraline for depression is similar to that of other antidepressants, and the differences are mostly confined to side effects. Sertraline is better tolerated than the older tricyclic antidepressants, and it may work better than fluoxetine for some subtypes of depression. Sertraline is effective for panic disorder, social anxiety disorder, generalized anxiety disorder, and obsessive–compulsive disorder (OCD). However, for OCD, cognitive behavioral therapy, particularly in combination with sertraline, is a better treatment. Although approved for post-traumatic stress disorder, sertraline leads to only modest improvement in this condition.[10][11] Sertraline also alleviates the symptoms of premenstrual dysphoric disorder and can be used in sub-therapeutic doses or intermittently for its treatment.[12]
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˈsɜːrtrəˌliːn/ |
| Trade names | Zoloft, Lustral, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a697048 |
| License data |
|
| Pregnancy category |
|
| Addiction liability | None[3] |
| Routes of administration | By mouth |
| Drug class | Selective serotonin reuptake inhibitor |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 44% |
| Protein binding | 98.5% |
| Metabolism | Liver (primarily N-demethylation mainly by CYP2B6; also metabolism by CYP2C19, others)[4][5] |
| Metabolites | • Desmethylsertraline • Others (e.g., hydroxylated metabolites, glucuronide conjugates)[4] |
| Elimination half-life | • Sertraline: 26 hours (32 hours in females, 22 hours in males; range 13–45 hours)[4][6][7][8] • Desmethylsertraline: 62–104 hours[4] |
| Excretion | Urine (40–45%)[4] Feces (40–45%)[4] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H17Cl2N |
| Molar mass | 306.23 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Sertraline shares the common side effects and contraindications of other SSRIs, with high rates of nausea, diarrhea, insomnia, and sexual side effects, but it appears not to lead to much weight gain, and its effects on cognitive performance are mild. Similar to other antidepressants, the use of sertraline for depression may be associated with a higher rate of suicidal thoughts and behavior in people under the age of 25. It should not be used together with MAO inhibitor medication: this combination causes serotonin syndrome. Sertraline taken during pregnancy is associated with a significant increase in congenital heart defects in newborns.[13][14]
Sertraline was invented and developed by scientists at Pfizer and approved for medical use in the United States in 1991. It is on the World Health Organization's List of Essential Medicines.[15] It is available as a generic medication.[9] In 2016, sertraline was the most commonly prescribed psychiatric medication in the United States[16] and in 2020, it was the twelfth most commonly prescribed medication in the United States, with over 38 million prescriptions.[17][18]
Medical uses
Sertraline has been approved for major depressive disorder (MDD), obsessive–compulsive disorder (OCD), posttraumatic stress disorder (PTSD), premenstrual dysphoric disorder (PMDD), panic disorder, and social anxiety disorder (SAD). Sertraline is not approved for use in children except for those with OCD.[19]
Depression
Multiple controlled clinical trials established efficacy of sertraline for the treatment of depression. Sertraline is also an effective antidepressant in the routine clinical practice. Continued treatment with sertraline prevents both a relapse of the current depressive episode and future episodes (recurrence of depression).[20]
In several double-blind studies, sertraline was consistently more effective than placebo for dysthymia, a more chronic variety of depression, and comparable to imipramine in that respect. Sertraline also improves the depression of dysthymic patients to a greater degree than psychotherapy.[20]
Limited pediatric data also demonstrates reduction in depressive symptoms in the pediatric population though remains a second line therapy after fluoxetine.[21][22]
Comparison with other antidepressants
In general, sertraline efficacy is similar to that of other antidepressants.[23] For example, a meta-analysis of 12 new-generation antidepressants showed that sertraline and escitalopram are the best in terms of efficacy and acceptability in the acute-phase treatment of adults with depression.[24] Comparative clinical trials demonstrated that sertraline is similar in efficacy against depression to moclobemide,[25] nefazodone,[26] escitalopram, bupropion,[27] citalopram, fluvoxamine, paroxetine,[24] venlafaxine[28] and mirtazapine.[29] Sertraline may be more efficacious for the treatment of depression in the acute phase (first 4 weeks) than fluoxetine.[30]
There are differences between sertraline and some other antidepressants in their efficacy in the treatment of different subtypes of depression and in their adverse effects. For severe depression, sertraline is as good as clomipramine but is better tolerated.[28] Sertraline appears to work better in melancholic depression than fluoxetine, paroxetine, and mianserin and is similar to the tricyclic antidepressants such as amitriptyline and clomipramine.[23] In the treatment of depression accompanied by OCD, sertraline performs significantly better than desipramine on the measures of both OCD and depression.[20][31] Sertraline is equivalent to imipramine for the treatment of depression with co-morbid panic disorder, but it is better tolerated.[32] Compared with amitriptyline, sertraline offered a greater overall improvement in quality of life of depressed patients.[23]
Depression in elderly
Sertraline used for the treatment of depression in elderly (older than 60) patients is superior to placebo and comparable to another SSRI fluoxetine, and tricyclic antidepressants (TCAs) amitriptyline, nortriptyline and imipramine. Sertraline has much lower rates of adverse effects than these TCAs, with the exception of nausea, which occurs more frequently with sertraline. In addition, sertraline appears to be more effective than fluoxetine or nortriptyline in the older-than-70 subgroup.[33] Accordingly, a meta-analysis of antidepressants in older adults found that sertraline, paroxetine and duloxetine were better than placebo.[34] On the other hand, in a 2003 trial the effect size was modest, and there was no improvement in quality of life as compared to placebo.[35] With depression in dementia, there is no benefit of sertraline treatment compared to either placebo or mirtazapine.[36]
Obsessive–compulsive disorder
Sertraline is effective for the treatment of OCD in adults and children.[19][37] It was better tolerated and, based on intention-to-treat analysis, performed better than the gold standard of OCD treatment clomipramine.[38] Continuing sertraline treatment helps prevent relapses of OCD with long-term data supporting its use for up to 24 months.[39] It is generally accepted that the sertraline dosages necessary for the effective treatment of OCD are higher than the usual dosage for depression.[40] The onset of action is also slower for OCD than for depression. The treatment recommendation is to start treatment with a half of maximal recommended dose for at least two months. After that, the dose can be raised to the maximal recommended in the cases of unsatisfactory response.[41]
Cognitive behavioral therapy alone was superior to sertraline in both adults and children; however, the best results were achieved using a combination of these treatments.[42][43]
Panic disorder
Sertraline is superior to placebo for the treatment of panic disorder.[19] The response rate was independent of the dose. In addition to decreasing the frequency of panic attacks by about 80% (vs. 45% for placebo) and decreasing general anxiety, sertraline resulted in improvement of quality of life on most parameters. The patients rated as "improved" on sertraline reported better quality of life than the ones who "improved" on placebo. The authors of the study argued that the improvement achieved with sertraline is different and of a better quality than the improvement achieved with placebo.[44][45] Sertraline is equally effective for men and women,[45] and for patients with or without agoraphobia.[46] Previous unsuccessful treatment with benzodiazepines does not diminish its efficacy.[47] However, the response rate was lower for the patients with more severe panic.[46] Starting treatment simultaneously with sertraline and clonazepam, with subsequent gradual discontinuation of clonazepam, may accelerate the response.[48]
Double-blind comparative studies found sertraline to have the same effect on panic disorder as paroxetine or imipramine.[49] While imprecise, comparison of the results of trials of sertraline with separate trials of other anti-panic agents (clomipramine, imipramine, clonazepam, alprazolam, and fluvoxamine) indicates approximate equivalence of these medications.[44]
Other anxiety disorders
Sertraline has been successfully used for the treatment of social anxiety disorder.[50][51] All three major domains of the disorder (fear, avoidance, and physiological symptoms) respond to sertraline.[20] Maintenance treatment, after the response is achieved, prevents the return of the symptoms.[52] The improvement is greater among the patients with later, adult onset of the disorder.[53] In a comparison trial, sertraline was superior to exposure therapy, but patients treated with the psychological intervention continued to improve during a year-long follow-up, while those treated with sertraline deteriorated after treatment termination.[54] The combination of sertraline and cognitive behavioral therapy appears to be more effective in children and young people than either treatment alone.[55]
Sertraline has not been approved for the treatment of generalized anxiety disorder; however, several guidelines recommend it as a first-line medication referring to good quality controlled clinical trials.[56][32][39]
Premenstrual dysphoric disorder
Sertraline is effective in alleviating the symptoms of premenstrual dysphoric disorder (PMDD), a severe form of premenstrual syndrome.[57] Significant improvement was observed in 50–60% of cases treated with sertraline vs. 20–30% of cases on placebo. The improvement began during the first week of treatment, and in addition to mood, irritability, and anxiety, improvement was reflected in better family functioning, social activity and general quality of life. Work functioning and physical symptoms, such as swelling, bloating and breast tenderness, were less responsive to sertraline.[58][59] Taking sertraline only during the luteal phase, that is, the 12–14 days before menses, was shown to work as well as continuous treatment.[57] Continuous treatment with sub-therapeutic doses of sertraline (25 mg vs. usual 50–100 mg) is also effective.[60]
Other indications
Sertraline is approved for the treatment of post-traumatic stress disorder (PTSD).[19] National Institute of Clinical Excellence recommends it for patients who prefer drug treatment to a psychological one.[61] Other guidelines also suggest sertraline as a first-line option for pharmacological therapy.[62][32] When necessary, long-term pharmacotherapy can be beneficial.[62] There are both negative and positive clinical trial results for sertraline, which may be explained by the types of psychological traumas, symptoms, and comorbidities included in the various studies.[39] Positive results were obtained in trials that included predominantly women (75%) with a majority (60%) having physical or sexual assault as the traumatic event.[62] Contrary to the above suggestions, a meta-analysis of sertraline clinical trials for PTSD found it to be not significantly better than placebo.[10] Another meta-analysis relegated sertraline to the second line, proposing trauma focused psychotherapy as a first-line intervention. The authors noted that Pfizer had declined to submit the results of a negative trial for the inclusion into the meta-analysis making the results unreliable.[11]
Sertraline when taken daily can be useful for the treatment of premature ejaculation.[63] A disadvantage of sertraline is that it requires continuous daily treatment to delay ejaculation significantly.[64]
A 2019 systematic review suggested that sertraline may be a good way to control anger, irritability and hostility in depressed patients and patients with other comorbidities.[65]
Contraindications
Sertraline is contraindicated in individuals taking monoamine oxidase inhibitors or the antipsychotic pimozide. Sertraline concentrate contains alcohol and is therefore contraindicated with disulfiram. The prescribing information recommends that treatment of the elderly and patients with liver impairment "must be approached with caution". Due to the slower elimination of sertraline in these groups, their exposure to sertraline may be as high as three times the average exposure for the same dose.[19]
Side effects
Nausea, ejaculation failure, insomnia, diarrhea, dry mouth, somnolence, dizziness, tremor, headache, excessive sweating, fatigue, and decreased libido are the common adverse effects associated with sertraline with the greatest difference from placebo. Those that most often resulted in interruption of the treatment are nausea, diarrhea and insomnia.[19] The incidence of diarrhea is higher with sertraline – especially when prescribed at higher doses – in comparison with other SSRIs.[66]
Over more than six months of sertraline therapy for depression, people showed a nonsignificant weight increase of 0.1%.[67] Similarly, a 30-month-long treatment with sertraline for OCD resulted in a mean weight gain of 1.5% (1 kg).[68] Although the difference did not reach statistical significance, the average weight gain was lower for fluoxetine (1%) but higher for citalopram, fluvoxamine and paroxetine (2.5%). Of the sertraline group, 4.5% gained a large amount of weight (defined as more than 7% gain). This result compares favorably with placebo, where, according to the literature, 3–6% of patients gained more than 7% of their initial weight. The large weight gain was observed only among female members of the sertraline group; the significance of this finding is unclear because of the small size of the group.[68]
Over a two-week treatment of healthy volunteers, sertraline slightly improved verbal fluency but did not affect word learning, short-term memory, vigilance, flicker fusion time, choice reaction time, memory span, or psychomotor coordination.[69][70] In spite of lower subjective rating, that is, feeling that they performed worse, no clinically relevant differences were observed in the objective cognitive performance in a group of people treated for depression with sertraline for 1.5 years as compared to healthy controls.[71] In children and adolescents taking sertraline for six weeks for anxiety disorders, 18 out of 20 measures of memory, attention and alertness stayed unchanged. Divided attention was improved and verbal memory under interference conditions decreased marginally. Because of the large number of measures taken, it is possible that these changes were still due to chance.[72] The unique effect of sertraline on dopaminergic neurotransmission may be related to these effects on cognition and vigilance.[73][74]
Sertraline has a low level of exposure of an infant through the breast milk and is recommended as the preferred option for the antidepressant therapy of breast-feeding mothers.[75][76] There is 29–42% increase in congenital heart defects among children whose mothers were prescribed sertraline during pregnancy,[13][14] with sertraline use in the first trimester associated with 2.7-fold increase in septal heart defects.[13]
Abrupt interruption of sertraline treatment may result in withdrawal or discontinuation syndrome. Dizziness, insomnia, anxiety, agitation, and irritability are its common symptoms.[77] It typically occurs within a few days from drug discontinuation and lasts a few weeks.[78] The withdrawal symptoms for sertraline are less severe and frequent than for paroxetine, and more frequent than for fluoxetine.[77][78] In most cases symptoms are mild, short-lived, and resolve without treatment. More severe cases are often successfully treated by temporary reintroduction of the drug with a slower tapering off rate.[79]
Sertraline and SSRI antidepressants in general may be associated with bruxism and other movement disorders.[80][81] Sertraline appears to be associated with microscopic colitis, a rare condition of unknown etiology.[82]
Sexual
Like other SSRIs, sertraline is associated with sexual side effects, including sexual arousal disorder, erectile dysfunction and difficulty achieving orgasm. While nefazodone and bupropion do not have negative effects on sexual functioning, 67% of men on sertraline experienced ejaculation difficulties versus 18% before the treatment.[83] Sexual arousal disorder, defined as "inadequate lubrication and swelling for women and erectile difficulties for men", occurred in 12% of people on sertraline as compared with 1% of patients on placebo. The mood improvement resulting from the treatment with sertraline sometimes counteracted these side effects, so that sexual desire and overall satisfaction with sex stayed the same as before the sertraline treatment. However, under the action of placebo the desire and satisfaction slightly improved.[84] Some people continue experiencing sexual side effects after they stop taking SSRIs.[85]
Suicide
The FDA requires all antidepressants, including sertraline, to carry a boxed warning stating that antidepressants increase the risk of suicide in persons younger than 25 years. This warning is based on statistical analyses conducted by two independent groups of FDA experts that found a 100% increase of suicidal thoughts and behavior in children and adolescents, and a 50% increase – in the 18–24 age group.[86][87][88]
Suicidal ideation and behavior in clinical trials are rare. For the above analysis, the FDA combined the results of 295 trials of 11 antidepressants for psychiatric indications in order to obtain statistically significant results. Considered separately, sertraline use in adults decreased the odds of suicidal behavior with a marginal statistical significance by 37%[88] or 50%[87] depending on the statistical technique used. The authors of the FDA analysis note that "given the large number of comparisons made in this review, chance is a very plausible explanation for this difference".[87] The more complete data submitted later by the sertraline manufacturer Pfizer indicated increased suicidal behavior.[89] Similarly, the analysis conducted by the UK MHRA found a 50% increase of odds of suicide-related events, not reaching statistical significance, in the patients on sertraline as compared to the ones on placebo.[90][91]
Overdose
Acute overdosage is often manifested by emesis, lethargy, ataxia, tachycardia and seizures. Plasma, serum or blood concentrations of sertraline and norsertraline, its major active metabolite, may be measured to confirm a diagnosis of poisoning in hospitalized patients or to aid in the medicolegal investigation of fatalities.[92] As with most other SSRIs its toxicity in overdose is considered relatively low.[93][94]
Interactions
As with other SSRIs, sertraline may increase the risk of bleeding with NSAIDs (ibuprofen, naproxen, mefenamic acid), antiplatelet drugs, anticoagulants, omega-3 fatty acids, vitamin E, and garlic supplements due to sertraline's inhibitory effects on platelet aggregation via blocking serotonin transporters on platelets.[95] Sertraline, in particular, may potentially diminish the efficacy of levothyroxine.[96]
Sertraline is a moderate inhibitor of CYP2D6 and CYP2B6 in vitro.[7] Accordingly, in human trials it caused increased blood levels of CYP2D6 substrates such as metoprolol, dextromethorphan, desipramine, imipramine and nortriptyline, as well as the CYP3A4/CYP2D6 substrate haloperidol.[97][98][99] This effect is dose-dependent; for example, co-administration with 50 mg of sertraline resulted in 20% greater exposure to desipramine, while 150 mg of sertraline led to a 70% increase.[8][100] In a placebo-controlled study, the concomitant administration of sertraline and methadone caused a 40% increase in blood levels of the latter, which is primarily metabolized by CYP2B6.[101]
Sertraline had a slight inhibitory effect on the metabolism of diazepam, tolbutamide and warfarin, which are CYP2C9 or CYP2C19 substrates; the clinical relevance of this effect was unclear.[8] As expected from in vitro data, sertraline did not alter the human metabolism of the CYP3A4 substrates erythromycin, alprazolam, carbamazepine, clonazepam, and terfenadine; neither did it affect metabolism of the CYP1A2 substrate clozapine.[8][19][102][7]
Sertraline had no effect on the actions of digoxin and atenolol, which are not metabolized in the liver.[4] Case reports suggest that taking sertraline with phenytoin or zolpidem may induce sertraline metabolism and decrease its efficacy,[103][104] and that taking sertraline with lamotrigine may increase the blood level of lamotrigine, possibly by inhibition of glucuronidation.[105]
CYP2C19 inhibitor esomeprazole increased sertraline concentrations in blood plasma by approximately 40%.[106]
Clinical reports indicate that interaction between sertraline and the MAOIs isocarboxazid and tranylcypromine may cause serotonin syndrome. In a placebo-controlled study in which sertraline was co-administered with lithium, 35% of the subjects experienced tremors, while none of those taking placebo did.[8]
Sertraline may interact with grapefruit juice—see "Grapefruit–drug interactions".
Pharmacology
Pharmacodynamics
| Site | Ki (nM) | Species | References | |
|---|---|---|---|---|
| SERT | 0.15–3.3 | Human | [108][109][110] | |
| NET | 420–925 | Human | [108][109][110] | |
| DAT | 22–315 | Human | [108][109][110] | |
| 5-HT1A | >35,000 | Human | [111] | |
| 5-HT2A | 2,207 | Rat | [110] | |
| 5-HT2C | 2,298 | Pig | [110] | |
| α1A | 1900 | Human | [112] | |
| α1B | 3,500 | Human | [112] | |
| α1D | 2,500 | Human | [112] | |
| α2 | 477–4,100 | Human | [109][111] | |
| D2 | 10,700 | Human | [111] | |
| H1 | 24,000 | Human | [111] | |
| mACh | 427–2,100 | Human | [110][111][113] | |
| σ1 | 32–57 | Rat | [114][115] | |
| σ2 | 5,297 | Rat | [115] | |
| Values are Ki (nM), unless otherwise noted. The smaller the value, the more strongly the drug binds to or inhibits the site. | ||||
Sertraline is a selective serotonin reuptake inhibitor (SSRI). By binding serotonin transporter (SERT) it inhibits neuronal reuptake of serotonin and potentiates serotonergic activity in the central nervous system.[19] Over time, this leads to a downregulation of pre-synaptic 5-HT1A receptors, which is associated with an improvement in passive stress tolerance, and delayed downstream increase in expression of brain-derived neurotrophic factor (BDNF), which may contribute to a reduction in negative affective biases.[116][117] It does not significantly affect norepinephrine transporter (NET), serotonin, dopamine, adrenergic, histamine, acetylcholine, GABA or benzodiazepine receptors.[19]
Sertraline also shows relatively high activity as an inhibitor of the dopamine transporter (DAT)[108][118][119] and antagonist of the sigma σ1 receptor (but not the σ2 receptor).[114][115][120] However, sertraline affinity for its main target (SERT) is much greater than its affinity for σ1 receptor and DAT.[107][108][115][114] Although there could be a role for the σ1 receptor in the pharmacology of sertraline, the significance of this receptor in its actions is unclear.[23] Similarly, the clinical relevance of sertraline's blockade of the dopamine transporter is uncertain.[108]
Pharmacokinetics
Absorption
Following a single oral dose of sertraline, mean peak blood levels of sertraline occur between 4.5 and 8.4 hours.[4] Bioavailability is likely linear and dose-proportional over a dose range of 150 to 200 mg.[4] Concomitant intake of sertraline with food slightly increases sertraline peak levels and total exposure.[4] There is an approximate 2-fold accumulation of sertraline with continuous administration and steady-state levels are reached within one week.[4]
Distribution
Sertraline is highly plasma protein bound (98.5%) across a concentration range of 20 to 500 ng/mL.[4] Despite the high plasma protein binding, sertraline and its metabolite desmethylsertraline at respective tested concentrations of 300 ng/mL and 200 ng/mL were found not to interfere with the plasma protein binding of warfarin and propranolol, two other highly plasma protein-bound drugs.[4]
Metabolism
Sertraline is subject to extensive first-pass metabolism, as indicated by a small study of radiolabeled sertraline in which less than 5% of plasma radioactivity was unchanged sertraline in two males.[4] The principal metabolic pathway for sertraline is N-demethylation into desmethylsertraline (N-desmethylsertraline) mainly by CYP2B6.[4][5] Reduction, hydroxylation, and glucuronide conjugation of both sertraline and desmethylsertraline also occur.[4] Desmethylsertraline, while pharmacologically active, is substantially (50-fold) weaker than sertraline as a serotonin reuptake inhibitor and its influence on the clinical effects of sertraline is thought to be negligible.[4][109][121] Based on in vitro studies, sertraline is metabolized by multiple cytochrome 450 isoforms;[5][122] however, it appears that in the human body CYP2C19 plays the most important role, followed by CYP2B6.[123] In addition to the cytochrome P450 system, sertraline can be oxidatively deaminated in vitro by monoamine oxidases;[4] however, this metabolic pathway has never been studied in vivo.[5]
Elimination
The elimination half-life of sertraline is on average 26 hours, with a range of 13 to 45 hours.[4][8] The half-life of sertraline is longer in women (32 hours) than in men (22 hours), which leads to 1.5-fold higher exposure to sertraline in women compared to men.[8] The elimination half-life of desmethylsertraline is 62 to 104 hours.[4]
In a small study of two males, sertraline was excreted to similar degrees in urine and feces (40 to 45% each within 9 days).[4] Unchanged sertraline was not detectable in urine, whereas 12 to 14% unchanged sertraline was present in feces.[4]
Pharmacogenomics
CYP2C19 and CYP2B6 are thought to be the key cytochrome P450 enzymes involved in the metabolism of sertraline.[123] Relative to CYP2C19 normal (extensive) metabolizers, poor metabolizers have 2.7-fold higher levels of sertraline[124] and intermediate metabolizers have 1.4-fold higher levels.[125] In contrast, CYP2B6 poor metabolizers have 1.6-fold higher levels of sertraline and intermediate metabolizers have 1.2-fold higher levels.[123]
History
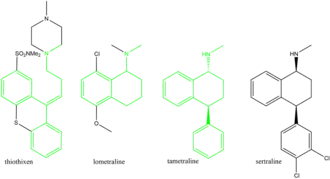
The history of sertraline dates back to the early 1970s, when Pfizer chemist Reinhard Sarges invented a novel series of psychoactive compounds, including lometraline, based on the structures of the neuroleptics thiothixene and pinoxepin.[126][127] Further work on these compounds led to tametraline, a norepinephrine and weaker dopamine reuptake inhibitor. Development of tametraline was soon stopped because of undesired stimulant effects observed in animals. A few years later, in 1977, pharmacologist Kenneth Koe, after comparing the structural features of a variety of reuptake inhibitors, became interested in the tametraline series. He asked another Pfizer chemist, Willard Welch, to synthesize some previously unexplored tametraline derivatives. Welch generated a number of potent norepinephrine and triple reuptake inhibitors, but to the surprise of the scientists, one representative of the generally inactive cis-analogs was a serotonin reuptake inhibitor. Welch then prepared stereoisomers of this compound, which were tested in vivo by animal behavioral scientist Albert Weissman. The most potent and selective (+)-isomer was taken into further development and eventually named sertraline. Weissman and Koe recalled that the group did not set up to produce an antidepressant of the SSRI type—in that sense their inquiry was not "very goal driven", and the discovery of the sertraline molecule was serendipitous. According to Welch, they worked outside the mainstream at Pfizer, and even "did not have a formal project team". The group had to overcome initial bureaucratic reluctance to pursue sertraline development, as Pfizer was considering licensing an antidepressant candidate from another company.[126][128][129]
Sertraline was approved by the US Food and Drug Administration (FDA) in 1991 based on the recommendation of the Psychopharmacological Drugs Advisory Committee; it had already become available in the United Kingdom the previous year.[130] The FDA committee achieved a consensus that sertraline was safe and effective for the treatment of major depression. During the discussion, Paul Leber, the director of the FDA Division of Neuropharmacological Drug Products, noted that granting approval was a "tough decision", since the treatment effect on outpatients with depression had been "modest to minimal". Other experts emphasized that the drug's effect on inpatients had not differed from placebo and criticized poor design of the clinical trials by Pfizer.[131] For example, 40% of participants dropped out of the trials, significantly decreasing their validity.[132]
Until 2002, sertraline was only approved for use in adults ages 18 and over; that year, it was approved by the FDA for use in treating children aged 6 or older with severe OCD. In 2003, the UK Medicines and Healthcare products Regulatory Agency issued a guidance that, apart from fluoxetine (Prozac), SSRIs are not suitable for the treatment of depression in patients under 18.[133][134] However, sertraline can still be used in the UK for the treatment of OCD in children and adolescents.[135] In 2005, the FDA added a boxed warning concerning pediatric suicidal behavior to all antidepressants, including sertraline. In 2007, labeling was again changed to add a warning regarding suicidal behavior in young adults ages 18 to 24.[136]
Society and culture
Other uses
Sertraline may be useful to treat murine Zaire ebolavirus (murine EBOV).[139] The World Health Organization (WHO) considers this a promising area of research.[139]
Lass-Flörl et al., 2003 finds it significantly inhibits phospholipase B in the fungal genus Candida, reducing virulence.[140]
Sertraline is also a very effective leishmanicide.[141] Specifically, Palit & Ali 2008 find that sertraline kills almost all promastigotes of Leishmania donovani.[141]
Sertraline is strongly antibacterial against some species.[141]
References
- "Sertraline international". Drugs.com. Retrieved 11 May 2015.
- "Sertraline (Zoloft) Use During Pregnancy". Drugs.com. 4 May 2020. Retrieved 17 May 2020.
- Hubbard JR, Martin PR (2001). Substance Abuse in the Mentally and Physically Disabled. CRC Press. p. 26. ISBN 9780824744977.
- Sertraline FDA Label Last updated May 2014
- Obach RS, Cox LM, Tremaine LM (February 2005). "Sertraline is metabolized by multiple cytochrome P450 enzymes, monoamine oxidases, and glucuronyl transferases in human: an in vitro study". Drug Metabolism and Disposition. 33 (2): 262–70. doi:10.1124/dmd.104.002428. PMID 15547048. S2CID 7254643.
- Brunton L, Chabner B, Knollman B (2010). Goodman and Gilman's The Pharmacological Basis of Therapeutics (Twelfth ed.). McGraw Hill Professional. ISBN 978-0-07-176939-6.
- Obach RS, Walsky RL, Venkatakrishnan K, Gaman EA, Houston JB, Tremaine LM (January 2006). "The utility of in vitro cytochrome P450 inhibition data in the prediction of drug–drug interactions". The Journal of Pharmacology and Experimental Therapeutics. 316 (1): 336–48. doi:10.1124/jpet.105.093229. PMID 16192315. S2CID 12975686.
- DeVane CL, Liston HL, Markowitz JS (2002). "Clinical pharmacokinetics of sertraline". Clin Pharmacokinet. 41 (15): 1247–66. doi:10.2165/00003088-200241150-00002. PMID 12452737. S2CID 28720641.
- "Sertraline Hydrochloride". Drugs.com. The American Society of Health-System Pharmacists. Retrieved 8 January 2018.
- Hoskins M, Pearce J, Bethell A, Dankova L, Barbui C, Tol WA, van Ommeren M, de Jong J, Seedat S, Chen H, Bisson JI (February 2015). "Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis". Br J Psychiatry. 206 (2): 93–100. doi:10.1192/bjp.bp.114.148551. PMID 25644881.
- Lee DJ, Schnitzlein CW, Wolf JP, Vythilingam M, Rasmusson AM, Hoge CW (September 2016). "Psychotherapy Versus Pharmacotherapy for Posttraumatic Stress Disorder: Systemic Review and Meta-Analyses to Determine First-Line Treatments". Depression and Anxiety. 33 (9): 792–806. doi:10.1002/da.22511. PMID 27126398. S2CID 20190202.
- Yonkers KA, Kornstein SG, Gueorguieva R, Merry B, Van Steenburgh K, Altemus M (October 2015). "Symptom-Onset Dosing of Sertraline for the Treatment of Premenstrual Dysphoric Disorder: A Randomized Clinical Trial". JAMA Psychiatry. 72 (10): 1037–1044. doi:10.1001/jamapsychiatry.2015.1472. PMC 4811029. PMID 26351969.
- Gao SY, Wu QJ, Sun C, Zhang TN, Shen ZQ, Liu CX, Gong TT, Xu X, Ji C, Huang DH, Chang Q, Zhao YH (November 2018). "Selective serotonin reuptake inhibitor use during early pregnancy and congenital malformations: a systematic review and meta-analysis of cohort studies of more than 9 million births". BMC Med. 16 (1): 205. doi:10.1186/s12916-018-1193-5. PMC 6231277. PMID 30415641.
- De Vries C, Gadzhanova S, Sykes MJ, Ward M, Roughead E (March 2021). "A Systematic Review and Meta-Analysis Considering the Risk for Congenital Heart Defects of Antidepressant Classes and Individual Antidepressants". Drug Saf. 44 (3): 291–312. doi:10.1007/s40264-020-01027-x. ISSN 0114-5916. PMID 33354752. S2CID 229357583.
- World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- Grohol JM (12 October 2017). "Top 25 Psychiatric Medications for 2016". Psych Central. Retrieved 22 October 2018.
- "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- "Sertraline - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.
- "DailyMed - ZOLOFT- sertraline hydrochloride tablet, film coated ZOLOFT- sertraline hydrochloride solution, concentrate".
- Sheehan DV, Kamijima K (March 2009). "An evidence-based review of the clinical use of sertraline in mood and anxiety disorders". Int Clin Psychopharmacol. 24 (2): 43–60. doi:10.1097/yic.0b013e3282f4b616. PMID 21456103.
- Zuckerbrot RA, Cheung A, Jensen PS, Stein RE, Laraque D (March 2018). "Guidelines for Adolescent Depression in Primary Care (GLAD-PC): Part I. Practice Preparation, Identification, Assessment, and Initial Management". Pediatrics. 141 (3). doi:10.1542/peds.2017-4081. PMID 29483200. S2CID 3591358.
- Depression in children and young people: identifification and management: NICE Guideline (PDF). National Institute for Health and Care Excellence (NICE). 25 June 2019.
- MacQueen G, Born L, Steiner M (2001). "The selective serotonin reuptake inhibitor sertraline: its profile and use in psychiatric disorders". CNS Drug Reviews. 7 (1): 1–24. doi:10.1111/j.1527-3458.2001.tb00188.x. PMC 6741657. PMID 11420570.
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, Watanabe N, Nakagawa A, Omori IM, McGuire H, Tansella M, Barbui C (February 2009). "Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis". Lancet. 373 (9665): 746–58. doi:10.1016/S0140-6736(09)60046-5. PMID 19185342. S2CID 35858125.; Lay summary in: Kahn M (28 January 2009). "Zoloft, Lexapro best new antidepressants-study". Reuters.
- Papakostas GI, Fava M (October 2006). "A metaanalysis of clinical trials comparing moclobemide with selective serotonin reuptake inhibitors for the treatment of major depressive disorder". Canadian Journal of Psychiatry. 51 (12): 783–90. doi:10.1177/070674370605101208. PMID 17168253.
- Feiger A, Kiev A, Shrivastava RK, Wisselink PG, Wilcox CS (1996). "Nefazodone versus sertraline in outpatients with major depression: focus on efficacy, tolerability, and effects on sexual function and satisfaction". The Journal of Clinical Psychiatry. 57. 57 (Suppl 2): 53–62. PMID 8626364.
- Kavoussi RJ, Segraves RT, Hughes AR, Ascher JA, Johnston JA (December 1997). "Double-blind comparison of bupropion sustained release and sertraline in depressed outpatients". The Journal of Clinical Psychiatry. 58 (12): 532–7. doi:10.4088/JCP.v58n1204. PMID 9448656.
- Nemeroff CB (2007). "The burden of severe depression: a review of diagnostic challenges and treatment alternatives". J Psychiatr Res. 41 (3–4): 189–206. doi:10.1016/j.jpsychires.2006.05.008. PMID 16870212.
- For the review, see:Hansen RA, Gartlehner G, Lohr KN, Gaynes BN, Carey TS (September 2005). "Efficacy and safety of second-generation antidepressants in the treatment of major depressive disorder". Annals of Internal Medicine. 143 (6): 415–26. doi:10.7326/0003-4819-143-6-200509200-00006. PMID 16172440. S2CID 10321621.
- Cipriani A, La Ferla T, Furukawa TA, Signoretti A, Nakagawa A, Churchill R, McGuire H, Barbui C (April 2010). "Sertraline versus other antidepressive agents for depression". The Cochrane Database of Systematic Reviews (4): CD006117. doi:10.1002/14651858.CD006117.pub4. PMC 4163971. PMID 20393946.
- Cleare A, Pariante CM, Young AH, Anderson IM, Christmas D, Cowen PJ, Dickens C, Ferrier IN, Geddes J, Gilbody S, Haddad PM, Katona C, Lewis G, Malizia A, McAllister-Williams RH, Ramchandani P, Scott J, Taylor D, Uher R (May 2015). "Evidence-based guidelines for treating depressive disorders with antidepressants: A revision of the 2008 British Association for Psychopharmacology guidelines". J Psychopharmacol. 29 (5): 459–525. doi:10.1177/0269881115581093. PMID 25969470. S2CID 8142581.
- Bandelow B, Zohar J, Hollander E, Kasper S, Möller HJ (October 2002). "World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders". World J Biol Psychiatry. 3 (4): 171–99. doi:10.3109/15622970209150621. PMID 12516310.
- Muijsers RB, Plosker GL, Noble S (2002). "Sertraline: a review of its use in the management of major depressive disorder in elderly patients". Drugs & Aging. 19 (5): 377–92. doi:10.2165/00002512-200219050-00006. PMID 12093324.
- Thorlund K, Druyts E, Wu P, Balijepalli C, Keohane D, Mills E (2015). "Comparative efficacy and safety of selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors in older adults: a network meta-analysis". J Am Geriatr Soc. 19 (63): 1002–1009. doi:10.1111/jgs.13395. PMID 25945410. S2CID 19041877.
- Schneider LS, Nelson JC, Clary CM, Newhouse P, Krishnan KR, Shiovitz T, Weihs K, et al. (Sertraline Elderly Depression Study Group) (July 2003). "An 8-week multicenter, parallel-group, double-blind, placebo-controlled study of sertraline in elderly outpatients with major depression" (PDF). The American Journal of Psychiatry. 160 (7): 1277–85. doi:10.1176/appi.ajp.160.7.1277. PMID 12832242. S2CID 25936853. Archived from the original (PDF) on 15 November 2020.
- Banerjee S, Hellier J, Romeo R, Dewey M, Knapp M, Ballard C, Baldwin R, Bentham P, Fox C, Holmes C, Katona C, Lawton C, Lindesay J, Livingston G, McCrae N, Moniz-Cook E, Murray J, Nurock S, Orrell M, O'Brien J, Poppe M, Thomas A, Walwyn R, Wilson K, Burns A (February 2013). "Study of the use of antidepressants for depression in dementia: the HTA-SADD trial--a multicentre, randomised, double-blind, placebo-controlled trial of the clinical effectiveness and cost-effectiveness of sertraline and mirtazapine". Health Technology Assessment. 17 (7): 1–166. doi:10.3310/hta17070. PMC 4782811. PMID 23438937.
- Geller DA, Biederman J, Stewart SE, Mullin B, Martin A, Spencer T, Faraone SV (November 2003). "Which SSRI? A meta-analysis of pharmacotherapy trials in pediatric obsessive-compulsive disorder" (PDF). The American Journal of Psychiatry. 160 (11): 1919–28. doi:10.1176/appi.ajp.160.11.1919. PMID 14594734. S2CID 8711232.
- Flament MF, Bisserbe JC (1997). "Pharmacologic treatment of obsessive-compulsive disorder: comparative studies". The Journal of Clinical Psychiatry. 58. 58 (Suppl 12): 18–22. PMID 9393392.
- Katzman MA, Bleau P, Blier P, Chokka P, Kjernisted K, Van Ameringen M, Antony MM, Bouchard S, Brunet A, Flament M, Grigoriadis S, Mendlowitz S, O'Connor K, Rabheru K, Richter PM, Robichaud M, Walker JR (2014). "Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders". BMC Psychiatry. 14 (Suppl 1): S1. doi:10.1186/1471-244X-14-S1-S1. PMC 4120194. PMID 25081580.
- Math SB, Janardhan Reddy YC (19 July 2007). "Issues In The Pharmacological Treatment of Obsessive-Compulsive Disorder: First-Line Treatment Options for OCD". medscape.com. Retrieved 28 July 2009.
- Blier P, Habib R, Flament MF (June 2006). "Pharmacotherapies in the management of obsessive-compulsive disorder". Canadian Journal of Psychiatry. 51 (7): 417–30. doi:10.1177/070674370605100703. PMID 16838823. S2CID 17133521.
- Pediatric OCD Treatment Study (POTS) Team (October 2004). "Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial". JAMA. 292 (16): 1969–76. doi:10.1001/jama.292.16.1969. PMID 15507582.
- Sousa MB, Isolan LR, Oliveira RR, Manfro GG, Cordioli AV (July 2006). "A randomized clinical trial of cognitive-behavioral group therapy and sertraline in the treatment of obsessive-compulsive disorder" (PDF). The Journal of Clinical Psychiatry. 67 (7): 1133–9. doi:10.4088/JCP.v67n0717. PMID 16889458. S2CID 25130472. Archived from the original (PDF) on 12 February 2020.
- Hirschfeld RM (2000). "Sertraline in the treatment of anxiety disorders". Depression and Anxiety. 11 (4): 139–57. doi:10.1002/1520-6394(2000)11:4<139::AID-DA1>3.0.CO;2-C. PMID 10945134. S2CID 25572278.
- Clayton AH, Stewart RS, Fayyad R, Clary CM (May 2006). "Sex differences in clinical presentation and response in panic disorder: pooled data from sertraline treatment studies". Archives of Women's Mental Health. 9 (3): 151–7. doi:10.1007/s00737-005-0111-y. PMID 16292466. S2CID 20606054.
- Pollack MH, Rapaport MH, Clary CM, Mardekian J, Wolkow R (December 2000). "Sertraline treatment of panic disorder: response in patients at risk for poor outcome". J Clin Psychiatry. 61 (12): 922–7. doi:10.4088/JCP.v61n1206. PMID 11206597.
- Rapaport MH, Pollack MH, Clary CM, Mardekian J, Wolkow R (February 2001). "Panic disorder and response to sertraline: the effect of previous treatment with benzodiazepines". J Clin Psychopharmacol. 21 (1): 104–7. doi:10.1097/00004714-200102000-00019. PMID 11199932. S2CID 13442642.
- Amrein R, Levitan M (2016). "Benzodiazepines in panic disorder". In Nardi AE (ed.). Panic disorder: Neurobiological and treatment aspects. Springer International Publishing. pp. 237–253. doi:10.1007/978-3-319-12538-1_16. ISBN 978-3-319-12537-4.
- Freire RC, Hallak JE, Crippa JA, Nardi AE (June 2011). "New treatment options for panic disorder: clinical trials from 2000 to 2010". Expert Opin Pharmacother. 12 (9): 1419–28. doi:10.1517/14656566.2011.562200. PMID 21342080. S2CID 207479015.
- Hansen RA, Gaynes BN, Gartlehner G, Moore CG, Tiwari R, Lohr KN (May 2008). "Efficacy and tolerability of second-generation antidepressants in social anxiety disorder". International Clinical Psychopharmacology. 23 (3): 170–9. doi:10.1097/YIC.0b013e3282f4224a. PMC 2657552. PMID 18408531.
- Davidson JR (2006). "Pharmacotherapy of social anxiety disorder: what does the evidence tell us?". The Journal of Clinical Psychiatry. 67 (Suppl 12): 20–6. doi:10.1016/j.genhosppsych.2005.07.002. PMID 17092192.
- Stein MB, Stein DJ (March 2008). "Social anxiety disorder". Lancet. 371 (9618): 1115–25. doi:10.1016/S0140-6736(08)60488-2. hdl:10983/15923. PMID 18374843. S2CID 29814976.
- Herpertz SC, Zanarini M, Schulz CS, Siever L, Lieb K, Möller HJ (2007). "World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of personality disorders". World J Biol Psychiatry. 8 (4): 212–44. doi:10.1080/15622970701685224. PMID 17963189. S2CID 14077861.
- Hollon SD, Stewart MO, Strunk D (2006). "Enduring effects for cognitive behavior therapy in the treatment of depression and anxiety". Annu Rev Psychol. 57: 285–315. doi:10.1146/annurev.psych.57.102904.190044. PMID 16318597.
- Mandrioli R, Mercolini L, Raggi MA (November 2013). "Evaluation of the pharmacokinetics, safety and clinical efficacy of sertraline used to treat social anxiety". Expert Opin Drug Metab Toxicol. 9 (11): 1495–505. doi:10.1517/17425255.2013.816675. PMID 23834458. S2CID 658089.
- "www.nice.org.uk".
- Marjoribanks J, Brown J, O'Brien PM, Wyatt K (June 2013). "Selective serotonin reuptake inhibitors for premenstrual syndrome" (PDF). The Cochrane Database of Systematic Reviews. 6 (6): CD001396. doi:10.1002/14651858.cd001396.pub3. PMC 7073417. PMID 23744611.
- Pearlstein T (2002). "Selective serotonin reuptake inhibitors for premenstrual dysphoric disorder: the emerging gold standard?". Drugs. 62 (13): 1869–85. doi:10.2165/00003495-200262130-00004. PMID 12215058. S2CID 46974228.
- Ackermann RT, Williams JW (April 2002). "Rational treatment choices for non-major depressions in primary care: an evidence-based review". Journal of General Internal Medicine. 17 (4): 293–301. doi:10.1046/j.1525-1497.2002.10350.x. PMC 1495030. PMID 11972726.
- Hantsoo L, Epperson CN (November 2015). "Premenstrual Dysphoric Disorder: Epidemiology and Treatment". Curr Psychiatry Rep. 17 (11): 87. doi:10.1007/s11920-015-0628-3. PMC 4890701. PMID 26377947.
- "www.nice.org.uk".
- Davis LL, Frazier EC, Williford RB, Newell JM (2006). "Long-term pharmacotherapy for post-traumatic stress disorder". CNS Drugs. 20 (6): 465–76. doi:10.2165/00023210-200620060-00003. PMID 16734498. S2CID 35429551.
- Abdel-Hamid IA (September 2006). "Pharmacologic treatment of rapid ejaculation: levels of evidence-based review". Current Clinical Pharmacology. 1 (3): 243–54. doi:10.2174/157488406778249352. PMID 18666749.
- Waldinger MD (November 2007). "Premature ejaculation: state of the art". The Urologic Clinics of North America. 34 (4): 591–9, vii–viii. doi:10.1016/j.ucl.2007.08.011. PMID 17983899.
- Romero-Martínez Á, Murciano-Martí S, Moya-Albiol L (May 2019). "Is Sertraline a Good Pharmacological Strategy to Control Anger? Results of a Systematic Review". Behavioral Sciences. 9 (5): 57. doi:10.3390/bs9050057. PMC 6562745. PMID 31126061.
- Sanchez C, Reines EH, Montgomery SA (July 2014). "A comparative review of escitalopram, paroxetine, and sertraline: Are they all alike?". International Clinical Psychopharmacology. 29 (4): 185–96. doi:10.1097/YIC.0000000000000023. PMC 4047306. PMID 24424469.
- Fava M, Judge R, Hoog SL, Nilsson ME, Koke SC (November 2000). "Fluoxetine versus sertraline and paroxetine in major depressive disorder: changes in weight with long-term treatment". The Journal of Clinical Psychiatry. 61 (11): 863–7. doi:10.4088/JCP.v61n1109. PMID 11105740.
- Maina G, Albert U, Salvi V, Bogetto F (October 2004). "Weight gain during long-term treatment of obsessive-compulsive disorder: a prospective comparison between serotonin reuptake inhibitors". The Journal of Clinical Psychiatry. 65 (10): 1365–71. doi:10.4088/JCP.v65n1011. PMID 15491240.
- Schmitt JA, Kruizinga MJ, Riedel WJ (September 2001). "Non-serotonergic pharmacological profiles and associated cognitive effects of serotonin reuptake inhibitors". Journal of Psychopharmacology. 15 (3): 173–9. doi:10.1177/026988110101500304. PMID 11565624. S2CID 26017110.
- Siepmann M, Grossmann J, Mück-Weymann M, Kirch W (July 2003). "Effects of sertraline on autonomic and cognitive functions in healthy volunteers". Psychopharmacology. 168 (3): 293–8. doi:10.1007/s00213-003-1448-4. PMID 12692706. S2CID 19178740.
- Gorenstein C, de Carvalho SC, Artes R, Moreno RA, Marcourakis T (March 2006). "Cognitive performance in depressed patients after chronic use of antidepressants". Psychopharmacology. 185 (1): 84–92. doi:10.1007/s00213-005-0274-2. PMID 16485140. S2CID 594353.
- Günther T, Holtkamp K, Jolles J, Herpertz-Dahlmann B, Konrad K (August 2005). "The influence of sertraline on attention and verbal memory in children and adolescents with anxiety disorders". Journal of Child and Adolescent Psychopharmacology. 15 (4): 608–18. CiteSeerX 10.1.1.536.6334. doi:10.1089/cap.2005.15.608. PMID 16190792.
- Borkowska A, Pilaczyńska E, Araszkiewicz A, Rybakowski J (2002). "[The effect of sertraline on cognitive functions in patients with obsessive-compulsive disorder]". Psychiatria Polska. 36 (6 Suppl): 289–95. PMID 12647451.
- Schmitt JA, Ramaekers JG, Kruizinga MJ, van Boxtel MP, Vuurman EF, Riedel WJ (September 2002). "Additional dopamine reuptake inhibition attenuates vigilance impairment induced by serotonin reuptake inhibition in man". Journal of Psychopharmacology. 16 (3): 207–14. doi:10.1177/026988110201600303. PMID 12236626. S2CID 25351919.
- Lattimore KA, Donn SM, Kaciroti N, Kemper AR, Neal CR, Vazquez DM (September 2005). "Selective serotonin reuptake inhibitor (SSRI) use during pregnancy and effects on the fetus and newborn: a meta-analysis". Journal of Perinatology. 25 (9): 595–604. doi:10.1038/sj.jp.7211352. PMID 16015372.
- McAllister-Williams RH, Baldwin DS, Cantwell R, Easter A, Gilvarry E, Glover V, Green L, Gregoire A, Howard LM, Jones I, Khalifeh H, Lingford-Hughes A, McDonald E, Micali N, Pariante CM, Peters L, Roberts A, Smith NC, Taylor D, Wieck A, Yates LM, Young AH (May 2017). "British Association for Psychopharmacology consensus guidance on the use of psychotropic medication preconception, in pregnancy and postpartum 2017" (PDF). J Psychopharmacol. 31 (5): 519–552. doi:10.1177/0269881117699361. PMID 28440103. S2CID 3335470.
- Renoir T (2013). "Selective serotonin reuptake inhibitor antidepressant treatment discontinuation syndrome: a review of the clinical evidence and the possible mechanisms involved". Front Pharmacol. 4: 45. doi:10.3389/fphar.2013.00045. PMC 3627130. PMID 23596418.
- Fava GA, Gatti A, Belaise C, Guidi J, Offidani E (2015). "Withdrawal Symptoms after Selective Serotonin Reuptake Inhibitor Discontinuation: A Systematic Review". Psychother Psychosom. 84 (2): 72–81. doi:10.1159/000370338. PMID 25721705.
- Warner CH, Bobo W, Warner C, Reid S, Rachal J (August 2006). "Antidepressant discontinuation syndrome". American Family Physician. 74 (3): 449–56. PMID 16913164.
- Revet A, Montastruc F, Roussin A, Raynaud JP, Lapeyre-Mestre M, Nguyen TT (June 2020). "Antidepressants and movement disorders: a postmarketing study in the world pharmacovigilance database". BMC Psychiatry. 20 (1): 308. doi:10.1186/s12888-020-02711-z. PMC 7298955. PMID 32546134.
- Garrett AR, Hawley JS (April 2018). "SSRI-associated bruxism: A systematic review of published case reports". Neurology. Clinical Practice. 8 (2): 135–141. doi:10.1212/CPJ.0000000000000433. PMC 5914744. PMID 29708207.
- Shor J, Churrango G, Hosseini N, Marshall C (2019). "Management of microscopic colitis: challenges and solutions". Clin Exp Gastroenterol. 12: 111–120. doi:10.2147/CEG.S165047. PMC 6398419. PMID 30881078.
- Ferguson JM (2001). "The effects of antidepressants on sexual functioning in depressed patients: a review". The Journal of Clinical Psychiatry. 62. 62 (Suppl 3): 22–34. PMID 11229450.
- Croft H, Settle E, Houser T, Batey SR, Donahue RM, Ascher JA (April 1999). "A placebo-controlled comparison of the antidepressant efficacy and effects on sexual functioning of sustained-release bupropion and sertraline". Clinical Therapeutics. 21 (4): 643–58. doi:10.1016/S0149-2918(00)88317-4. PMID 10363731.
- Bala A, Nguyen HM, Hellstrom WJ (January 2018). "Post-SSRI Sexual Dysfunction: A Literature Review". Sexual Medicine Reviews. 6 (1): 29–34. doi:10.1016/j.sxmr.2017.07.002. PMID 28778697.
- Levenson M, Holland C. "Antidepressants and Suicidality in Adults: Statistical Evaluation. (Presentation at Psychopharmacologic Drugs Advisory Committee; December 13, 2006)". FDA. Retrieved 11 July 2008.
- Stone MB, Jones ML (17 November 2006). "Clinical review: relationship between antidepressant drugs and suicidality in adults" (PDF). Overview for December 13 Meeting of Psychopharmacologic Drugs Advisory Committee (PDAC). FDA. pp. 11–74. Retrieved 11 July 2008.
- Levenson M, Holland C (17 November 2006). "Statistical Evaluation of Suicidality in Adults Treated with Antidepressants" (PDF). Overview for December 13 Meeting of Psychopharmacologic Drugs Advisory Committee (PDAC). FDA. pp. 75–140. Retrieved 11 July 2008.
- Pfizer Inc. (30 November 2006). "Memorandum from Pfizer Global Pharmaceuticals Re: DOCKET: 2006N-0414 –"Suicidality data from adult antidepressant trials" Background package for December 13 Advisory Committee" (PDF). FDA DOCKET 2006N-0414. FDA. Retrieved 11 July 2008.
- "Report of the CSM expert working group on the safety of selective serotonin reuptake inhibitor antidepressants" (PDF). MHRA. December 2004. Retrieved 11 July 2008.
- Gunnell D, Saperia J, Ashby D (February 2005). "Selective serotonin reuptake inhibitors (SSRIs) and suicide in adults: meta-analysis of drug company data from placebo controlled, randomised controlled trials submitted to the MHRA's safety review". BMJ. 330 (7488): 385. doi:10.1136/bmj.330.7488.385. PMC 549105. PMID 15718537.
- Baselt R (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, California: Biomedical Publications. pp. 1399–1400.
- Taylor D, Paton C, Shitij K (2012). The Maudsley prescribing guidelines in psychiatry. West Sussex: Wiley-Blackwell. ISBN 978-0-470-97948-8.
- White N, Litovitz T, Clancy C (December 2008). "Suicidal antidepressant overdoses: a comparative analysis by antidepressant type". Journal of Medical Toxicology. 4 (4): 238–50. doi:10.1007/BF03161207. PMC 3550116. PMID 19031375.
- "UpToDate". www.uptodate.com. Retrieved 10 August 2022.
- AbbVie (February 2017). "SYNTHROID® (levothyroxine sodium) tablets, for oral use" (PDF). U.S. Food & Drug Administration. Retrieved 10 August 2022.
{{cite web}}: CS1 maint: url-status (link) - Ozdemir V, Naranjo CA, Herrmann N, Shulman RW, Sellers EM, Reed K, Kalow W (February 1998). "The extent and determinants of changes in CYP2D6 and CYP1A2 activities with therapeutic doses of sertraline". Journal of Clinical Psychopharmacology. 18 (1): 55–61. doi:10.1097/00004714-199802000-00009. PMID 9472843.
- Alfaro CL, Lam YW, Simpson J, Ereshefsky L (April 1999). "CYP2D6 status of extensive metabolizers after multiple-dose fluoxetine, fluvoxamine, paroxetine, or sertraline". Journal of Clinical Psychopharmacology. 19 (2): 155–63. doi:10.1097/00004714-199904000-00011. PMID 10211917.
- Alfaro CL, Lam YW, Simpson J, Ereshefsky L (January 2000). "CYP2D6 inhibition by fluoxetine, paroxetine, sertraline, and venlafaxine in a crossover study: intraindividual variability and plasma concentration correlations". Journal of Clinical Pharmacology. 40 (1): 58–66. doi:10.1177/00912700022008702. PMID 10631623.
- Preskorn SH, Greenblatt DJ, Flockhart D, Luo Y, Perloff ES, Harmatz JS, Baker B, Klick-Davis A, Desta Z, Burt T (February 2007). "Comparison of duloxetine, escitalopram, and sertraline effects on cytochrome P450 2D6 function in healthy volunteers". Journal of Clinical Psychopharmacology. 27 (1): 28–34. doi:10.1097/00004714-200702000-00005. PMID 17224709. S2CID 28468404.
- Hamilton SP, Nunes EV, Janal M, Weber L (2000). "The effect of sertraline on methadone plasma levels in methadone-maintenance patients". The American Journal on Addictions. 9 (1): 63–9. doi:10.1080/10550490050172236. PMID 10914294.
- DeVane CL, Donovan JL, Liston HL, Markowitz JS, Cheng KT, Risch SC, Willard L (February 2004). "Comparative CYP3A4 inhibitory effects of venlafaxine, fluoxetine, sertraline, and nefazodone in healthy volunteers". Journal of Clinical Psychopharmacology. 24 (1): 4–10. doi:10.1097/01.jcp.0000104908.75206.26. PMID 14709940. S2CID 25826168.
- Allard S, Sainati SM, Roth-Schechter BF (February 1999). "Coadministration of short-term zolpidem with sertraline in healthy women". Journal of Clinical Pharmacology. 39 (2): 184–91. doi:10.1177/00912709922007624. PMID 11563412. S2CID 35439672.
- Haselberger MB, Freedman LS, Tolbert S (April 1997). "Elevated serum phenytoin concentrations associated with coadministration of sertraline". Journal of Clinical Psychopharmacology. 17 (2): 107–9. doi:10.1097/00004714-199704000-00008. PMID 10950473.
- Kaufman KR, Gerner R (April 1998). "Lamotrigine toxicity secondary to sertraline". Seizure. 7 (2): 163–5. doi:10.1016/S1059-1311(98)80074-5. PMID 9627209. S2CID 35861342.
- Gjestad C, Westin AA, Skogvoll E, Spigset O (February 2015). "Effect of proton pump inhibitors on the serum concentrations of the selective serotonin reuptake inhibitors citalopram, escitalopram, and sertraline". Ther Drug Monit. 37 (1): 90–7. doi:10.1097/FTD.0000000000000101. PMC 4297217. PMID 24887634.
- Roth BL, Driscol J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- Tatsumi M, Groshan K, Blakely RD, Richelson E (December 1997). "Pharmacological profile of antidepressants and related compounds at human monoamine transporters". European Journal of Pharmacology. 340 (2–3): 249–58. doi:10.1016/s0014-2999(97)01393-9. PMID 9537821.
- Owens MJ, Morgan WN, Plott SJ, Nemeroff CB (December 1997). "Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites". The Journal of Pharmacology and Experimental Therapeutics. 283 (3): 1305–22. PMID 9400006.
- Owens MJ, Knight DL, Nemeroff CB (September 2001). "Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine". Biological Psychiatry. 50 (5): 345–50. doi:10.1016/s0006-3223(01)01145-3. PMID 11543737. S2CID 11247427.
- Cusack B, Nelson A, Richelson E (May 1994). "Binding of antidepressants to human brain receptors: focus on newer generation compounds". Psychopharmacology. 114 (4): 559–65. doi:10.1007/bf02244985. PMID 7855217. S2CID 21236268.
- Proudman RG, Pupo AS, Baker JG (August 2020). "The affinity and selectivity of α-adrenoceptor antagonists, antidepressants, and antipsychotics for the human α1A, α1B, and α1D-adrenoceptors". Pharmacol Res Perspect. 8 (4): e00602. doi:10.1002/prp2.602. PMC 7327383. PMID 32608144.
- Stanton T, Bolden-Watson C, Cusack B, Richelson E (June 1993). "Antagonism of the five cloned human muscarinic cholinergic receptors expressed in CHO-K1 cells by antidepressants and antihistaminics". Biochemical Pharmacology. 45 (11): 2352–4. doi:10.1016/0006-2952(93)90211-e. PMID 8100134.
- Albayrak Y, Hashimoto K (2017). "Sigma-1 Receptor Agonists and Their Clinical Implications in Neuropsychiatric Disorders". Sigma Receptors: Their Role in Disease and as Therapeutic Targets. Advances in Experimental Medicine and Biology. Vol. 964. pp. 153–161. doi:10.1007/978-3-319-50174-1_11. ISBN 978-3-319-50172-7. PMID 28315270.
- Hindmarch I, Hashimoto K (April 2010). "Cognition and depression: the effects of fluvoxamine, a sigma-1 receptor agonist, reconsidered". Human Psychopharmacology. 25 (3): 193–200. doi:10.1002/hup.1106. PMID 20373470. S2CID 26491662.
- Carhart-Harris RL, Nutt DJ (September 2017). "Serotonin and brain function: a tale of two receptors". Journal of Psychopharmacology. 31 (9): 1091–1120. doi:10.1177/0269881117725915. PMC 5606297. PMID 28858536.
- Harmer CJ, Duman RS, Cowen PJ (May 2017). "How do antidepressants work? New perspectives for refining future treatment approaches". The Lancet. Psychiatry. 4 (5): 409–418. doi:10.1016/S2215-0366(17)30015-9. PMC 5410405. PMID 28153641.
- Richelson E (May 2001). "Pharmacology of antidepressants". Mayo Clinic Proceedings. 76 (5): 511–27. doi:10.4065/76.5.511. PMID 11357798.
- Hemmings HC, Egan TD (2012). Pharmacology and Physiology for Anesthesia E-Book: Foundations and Clinical Application. Elsevier Health Sciences. pp. 183–. ISBN 978-1-4557-3793-2.
- Hashimoto K (September 2009). "Sigma-1 receptors and selective serotonin reuptake inhibitors: clinical implications of their relationship". Central Nervous System Agents in Medicinal Chemistry. 9 (3): 197–204. doi:10.2174/1871524910909030197. PMID 20021354.
- Sprouse J, Clarke T, Reynolds L, Heym J, Rollema H (April 1996). "Comparison of the effects of sertraline and its metabolite desmethylsertraline on blockade of central 5-HT reuptake in vivo". Neuropsychopharmacology. 14 (4): 225–231. doi:10.1016/0893-133X(95)00112-Q. PMID 8924190. S2CID 39841365.
- Kobayashi K, Ishizuka T, Shimada N, Yoshimura Y, Kamijima K, Chiba K (July 1999). "Sertraline N-demethylation is catalyzed by multiple isoforms of human cytochrome P-450 in vitro". Drug Metabolism and Disposition. 27 (7): 763–6. PMID 10383917.
- Saiz-Rodríguez M, Belmonte C, Román M, Ochoa D, Koller D, Talegón M, Ovejero-Benito MC, López-Rodríguez R, Cabaleiro T, Abad-Santos F (May 2018). "Effect of Polymorphisms on the Pharmacokinetics, Pharmacodynamics and Safety of Sertraline in Healthy Volunteers". Basic & Clinical Pharmacology & Toxicology. 122 (5): 501–511. doi:10.1111/bcpt.12938. PMID 29136336.
- Bråten LS, Haslemo T, Jukic MM, Ingelman-Sundberg M, Molden E, Kringen MK (February 2020). "Impact of CYP2C19 genotype on sertraline exposure in 1200 Scandinavian patients". Neuropsychopharmacology. 45 (3): 570–576. doi:10.1038/s41386-019-0554-x. PMC 6969041. PMID 31649299.
- Milosavljevic F, Bukvic N, Pavlovic Z, Miljevic C, Pešic V, Molden E, Ingelman-Sundberg M, Leucht S, Jukic MM (November 2020). "Association of CYP2C19 and CYP2D6 Poor and Intermediate Metabolizer Status With Antidepressant and Antipsychotic Exposure: A Systematic Review and Meta-analysis". JAMA Psychiatry. 78 (3): 270–280. doi:10.1001/jamapsychiatry.2020.3643. PMC 7702196. PMID 33237321.
- Welch WM (1995). Discovery and Development of Sertraline. Advances in Medicinal Chemistry. Vol. 3. pp. 113–148. doi:10.1016/S1067-5698(06)80005-2. ISBN 978-1-55938-798-9.
- Sarges R, Tretter JR, Tenen SS, Weissman A (September 1973). "5,8-Disubstituted 1-aminotetralins. A class of compounds with a novel profile of central nervous system activity". Journal of Medicinal Chemistry. 16 (9): 1003–11. doi:10.1021/jm00267a010. PMID 4795663.
- See also: Mullin R (2006). "ACS Award for Team Innovation". Chemical & Engineering News. 84 (5): 45–52. doi:10.1021/cen-v084n010.p045.
- A short blurb on the history of sertraline, see: Couzin J (July 2005). "The brains behind blockbusters". Science. 309 (5735): 728. doi:10.1126/science.309.5735.728. PMID 16051786. S2CID 45532935.
- Healy D (1999). The Antidepressant Era. Cambridge, Massachusetts: Harvard University Press. p. 168. ISBN 978-0-674-03958-2.
- "Minutes of the 33rd Meeting of Psychopharmacological Drugs Advisory Committee on November 19, 1990" (PDF). FDA. 1990. Retrieved 11 July 2008.
- See also:Fabre LF, Abuzzahab FS, Amin M, Claghorn JL, Mendels J, Petrie WM, et al. (November 1995). "Sertraline safety and efficacy in major depression: a double-blind fixed-dose comparison with placebo". Biological Psychiatry. 38 (9): 592–602. doi:10.1016/0006-3223(95)00178-8. PMID 8573661. S2CID 27253073.
- "Safety review of antidepressants used by children completed". MHRA. 10 December 2003. Archived from the original on 16 June 2008. Retrieved 11 July 2008.
- Boseley S (10 December 2003). "Drugs for depressed children banned". The Guardian. Retrieved 19 April 2007.
- "Overview of regulatory status and CSM advice relating to major depressive disorder (MDD) in children and adolescents". MHRA. Archived from the original on 2 August 2008. Retrieved 17 April 2008.
- Food and Drug Administration (2 May 2007). "FDA Proposes New Warnings About Suicidal Thinking, Behavior in Young Adults Who Take Antidepressant Medications". Food and Drug Administration. Retrieved 11 July 2008.
- Smith A (17 July 2006). "Pfizer needs more drugs". CNN. Retrieved 27 January 2007.
- Edney A (1 June 2020). "Zoloft in Short Supply as Prescriptions Soar During Pandemic". Bloomberg. Retrieved 3 June 2020.
-
- • Cardile AP, Warren TK, Martins KA, Reisler RB, Bavari S (January 2017). "Will There Be a Cure for Ebola?". Annual Review of Pharmacology and Toxicology. Annual Reviews. 57 (1): 329–348. doi:10.1146/annurev-pharmtox-010716-105055. PMID 27959624.
- • Johansen LM, DeWald LE, Shoemaker CJ, Hoffstrom BG, Lear-Rooney CM, Stossel A, et al. (June 2015). "A screen of approved drugs and molecular probes identifies therapeutics with anti-Ebola virus activity". Science Translational Medicine. American Association for the Advancement of Science (AAAS). 7 (290): 290ra89. doi:10.1126/scitranslmed.aaa5597. PMID 26041706. S2CID 88805622.
-
- • Lass-Flörl C, Ledochowski M, Fuchs D, Speth C, Kacani L, Dierich MP, et al. (January 2003). "Interaction of sertraline with Candida species selectively attenuates fungal virulence in vitro". FEMS Immunology and Medical Microbiology. Oxford University Press (Federation of European Microbiological Societies). 35 (1): 11–15. doi:10.1111/j.1574-695x.2003.tb00643.x. PMID 12589952. S2CID 11240443.
- • Schaller M, Borelli C, Korting HC, Hube B (November 2005). "Hydrolytic enzymes as virulence factors of Candida albicans". Mycoses. Blackwell Publishing. 48 (6): 365–377. doi:10.1111/j.1439-0507.2005.01165.x. PMID 16262871. S2CID 1356254.
- • Lyte M, ed. (2016). Microbial Endocrinology: Interkingdom Signaling in Infectious Disease and Health. Advances in Experimental Medicine and Biology. Vol. 874. Cham, Switzerland. pp. xiii+374. doi:10.1007/978-3-319-20215-0. ISBN 978-3-319-20215-0. OCLC 932167823. S2CID 7784624. ISBN 978-3-319-20214-3. ISBN 978-3-319-79299-6.: 349, 355
- • Cussotto S, Clarke G, Dinan TG, Cryan JF (May 2019). "Psychotropics and the Microbiome: a Chamber of Secrets…". Psychopharmacology. Springer Science and Business Media (European Behavioural Pharmacology Society (EBPS)). 236 (5): 1411–1432. doi:10.1007/s00213-019-5185-8. PMC 6598948. PMID 30806744. S2CID 71145305.
- Cussotto S, Clarke G, Dinan TG, Cryan JF (May 2019). "Psychotropics and the Microbiome: a Chamber of Secrets…". Psychopharmacology. Springer Science and Business Media (European Behavioural Pharmacology Society (EBPS)). 236 (5): 1411–1432. doi:10.1007/s00213-019-5185-8. PMC 6598948. PMID 30806744. S2CID 71145305.
External links
- "Sertraline". Drug Information Portal. US National Library of Medicine.
- "Sertraline hydrochloride". Drug Information Portal. US National Library of Medicine.