Nucleophilic substitution is a superset of reactions that involve an electron-rich nucleophile bonding to an electrophilic carbon atom and displacing a stable leaving group.
Generally, the form of a nucleophilic substitution reaction can be expressed as:
Nuc: + R-LG → R-Nuc + LG:
Note that the nucleophile (Nuc) uses a lone pair of electrons to form a bond with the carbon at R (which represents a carbon-containing structure), and the electrons from the R-LG bond end up attached to the leaving group (LG).
For a species to be classified as a nucleophile it must have a lone pair of electrons that can an empty atomic or molecular orbital. A wide variety of species can act as nucleophiles, from halide anions to water and more, depending on reaction conditions. This will be explained further in the detailed sections on unimolecular and bimolecular forms of nucleophilic substitution.
All leaving groups are species that are stable after breaking away from a molecule and bringing both electrons from the previous bond as a lone pair. Typically, strength of a leaving group's conjugate acid correlates with its lability.
Unimolecular Nucleophilic Substitution
In unimolecular nucleophilic substitution (SN1), a leaving group is replaced by a nucleophile in a two-step process. This process can be diagramed as:
R-LG → R+ + LG: (step 1)
Nuc: + R+ → R-Nuc (step 2)
The LG: species can have a neutral or negative charge, but the R group must be positive after it detaches. This leaves an empty p-orbital on the carbon, where the nucleophile (which can be either negative or neutral) can form a bond. The mechanism for SN1 is:
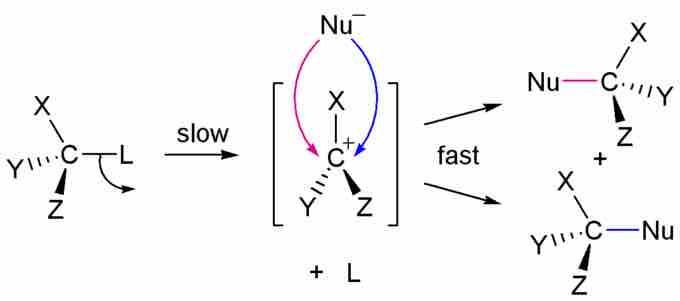
SN1 Mechanism
In SN1 reactions, the rate-determining step is the removal of the leaving group. This is typically performed at high temperatures, with polar solvents that can align their negative poles to stabilize the positive carbon (carbocation). To further polarize the conditions, these reactions are often performed in acidic solutions.
Nucleophiles in SN1 reactions need not to be as strong as those required for SN2 reactions, because the carbocation is extremely unstable and will accept any nearby electron-donor. Halide anions are effective in SN1, and even water can replace a leaving group (the water's O atom bonds to the carbon, and one of the H atoms is removed to form an alcohol). Ineffective nucleophiles in SN1 conditions are those that are bulky and/or are strong bases. If they are large, they will have difficulty accessing the carbocation's empty p-orbital. The stronger they are as bases, the more likely they will perform an elimination reaction instead of nucleophilic substitution.
Bimolecular Nucleophilic Substitution
Bimolecular nucleophilic substitution (SN2) occurs in a one-step process that can be modeled as:
Nuc: + R-LG → Nuc-R + LG:
Nucleophiles in SN2 reactions are often negative, but sometimes are neutral in charge. Leaving groups are almost always negative.
In SN2, the nucleophile "pushes" the leaving group off the carbon in the R group. The nucleophile can do this if its nonbonding electrons access the antibonding orbital of the R-LG bond. As this occurs, the bond order of R-LG decreases from 1 to 0, and the nucleophile forms a bond with carbon. The mechanism for SN2 is:
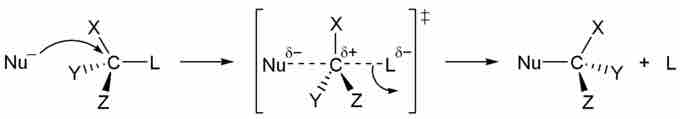
SN2 Mechanism
SN2 is known as a "backside" reaction: it always occurs opposite the R-LG bond. Thus, if the carbon involved in the SN2 reaction is a stereocenter, this stereocenter is inverted following the reaction.
Nucleophiles in SN2 reactions are typically stronger than in SN1, because they have to force the leaving group away. Conditions need not to be in acid, so basic nucleophiles such as amines can be used. With increasing polarizability (which goes hand-in-hand with increasing size of the nucleophilic atom) and basicity comes increasing nucleophilicity, although basicity and polarizability can have opposing effects (ex: iodide is less basic than fluoride but is larger).
Strong nucleophiles include the larger halides (I- and Br-), deprotonated thiols (RS-) and bisulfide (HS-), amides (NH2- or RNH-), azides (N3-), and cyanides (RCN-). As in SN1, SN2 reactions can compete with elimination (E2) depending on the basicity of the nucleophile and accessibility of the carbon electrophile. Lesser substitution on the carbon (more hydrogen and fewer carbon atoms attached) favors SN2.
Leaving groups for SN2 reactions can be similar to those used in SN1. The only criterion is that they are labile enough to be "pushed" away by the nucleophile.