Hydrocarbons are the simplest class of organic compounds and are composed solely of hydrogen and carbon. This class can be further divided into two groups: aliphatic hydrocarbons and aromatic hydrocarbons.
Aliphatic Hydrocarbons
Aliphatic hydrocarbons can be classified based on the structure and bonding of the carbon skeleton into one of three groups: alkanes, alkenes, and alkynes.
- Alkanes, or saturated hydrocarbons, are compounds that consist entirely of single bonds, so that each carbon atom is connected to four other atoms (either another carbon within the skeletal structure or a hydrogen atom). They can be described by the formula CnH2n+2. One simple example is methane, where n=1 and therefore has a chemical formula of CH4. Cycloalkanes are structures composed of single bonds where their carbon atoms are connected in a ring.
- Alkenes and alkynes are known as unsaturated hydrocarbons because some of the carbons are connected to fewer than four neighboring atoms. Alkenes contain at least one double bond, while alkynes contain at least one triple bond.
Aromatic Hydrocarbons
Aromatic hydrocarbons, or arenes, which contain a benzene ring, were originally named for their pleasant odors. A benzene ring is a ring of six carbons with alternating double and single bonds. As a result, the benzene has six hydrogens and the formula for a benzene molecule is C6H6. The benzene ring has resonance, meaning that the electron density is delocalized in the ring so that each bond is more similar to 1.5 bonds than either single or double bonds. These compounds possess special properties due to the delocalized electron density in benzene, including additional stabilization.
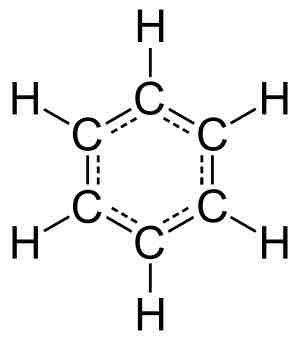
A benzene molecule
The benzene molecules and its derivatives are the basis for aromatic structures.
Many organic compounds belong in multiple categories. For example, a chemical structure can be both aromatic and contain an alkyne. Therefore, naming organic compounds can be quite challenging and complicated.
The study of hydrocarbons is particularly important to the fields of chemical and petroleum engineering, as a variety of hydrocarbons can be found in crude oil. This material can be processed to produce compounds for a number of applications. For example, the hydrocarbons in oil are burned in combustion to create power for cars, houses, and businesses.