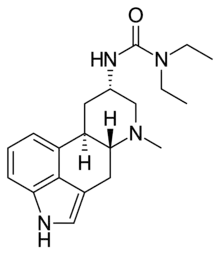
Terguride
 | |
| Clinical data | |
|---|---|
| Trade names | Teluron |
| Other names | Dironyl; Mysalfon; trans-Dihydrolisuride; Transdihydrolisuride; TDHL; SH-406; VUFB-6638; ZK-31224; N,N-Diethyl-N'-[(8α)-6-methylergolin-8-yl]urea |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.048.732 |
| Chemical and physical data | |
| Formula | C20H28N4O |
| Molar mass | 340.471 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Terguride (INN, JAN), sold under the brand name Teluron, is a serotonin receptor antagonist and dopamine receptor agonist of the ergoline family. It is approved for and used as a prolactin inhibitor in the treatment of hyperprolactinemia (high prolactin levels) in Japan.[1][2] Terguride is taken by mouth.
Pharmacology
Pharmacodynamics
Terguride acts as an agonist of the dopamine D2 receptor and as an antagonist of the serotonin 5-HT2A and 5-HT2B receptors, among other actions.
As an antagonist of the 5-HT2B receptor, terguride is not associated with cardiac valvulopathy.[3]
| Site | Affinity (Ki [nM]) | Efficacy (Emax [%]) | Action |
|---|---|---|---|
| D1 | 28 | ? | ? |
| D2S | 0.81 | 39 | Partial agonist |
| D2L | 1.1 | 0 | Silent antagonist |
| D3 | 1.0 | 36 | Partial agonist |
| D4 | 8.1 | 0 | Silent antagonist |
| D5 | 23 | ? | ? |
| 5-HT1A | 3.5 | 71 | Partial agonist |
| 5-HT1B | 257 | 37 | Partial agonist |
| 5-HT1D | 16 | 62 | Partial agonist |
| 5-HT2A | 4.8 | 49 | Partial agonist |
| 5-HT2B | 7.1 | 0 | Silent antagonist |
| 5-HT2C | 48 | 0 | Silent antagonist |
| 5-HT7 | 8–42 | ? | ? |
| α1A | 3.5 | 0 | Silent antagonist |
| α1B | 35 | ? | ? |
| α1D | 3.9 | ? | ? |
| α2A | 0.30 | 0 | Silent antagonist |
| α2B | 0.45 | 0 | Silent antagonist |
| α2C | 0.76 | 0 | Silent antagonist |
| α2D | 1.5 | ? | ? |
| β1 | 661 | ? | ? |
| β2 | 20 | ? | ? |
| H1 | 339 | ? | ? |
| M1 | >10,000 | ? | ? |
| Notes: All receptors are human except α2D-adrenergic, which is rat (no human counterpart), and 5-HT7, which was guinea pig.[4][7] | |||
Research
Serotonin stimulates the proliferation of pulmonary artery smooth muscle cells, and induces fibrosis in the wall of pulmonary arteries. Together, this causes vascular remodeling and narrowing of the pulmonary arteries. These changes result in increased vascular resistance and PAH. Due to the potential anti-proliferative and anti-fibrotic activity of terguride, this potential medicine could offer the hope of achieving reversal of pulmonary artery vascular remodeling and attenuation of disease progression.[8] In May 2008, terguride was granted orphan drug status for the treatment of pulmonary arterial hypertension.[9] In May 2010 Pfizer purchased worldwide rights for the drug.[10] However, development was discontinued in 2011.
References
- ↑ "List of 5HT3 receptor antagonists (5hydroxytryptamine receptor antagonists)". Archived from the original on 2016-03-03.
- ↑ "Terguride - AdisInsight".
- ↑ Zajdel P, Bednarski M, Sapa J, Nowak G (April 2015). "Ergotamine and nicergoline - facts and myths". Pharmacol Rep. 67 (2): 360–3. doi:10.1016/j.pharep.2014.10.010. PMID 25712664.
- 1 2 Millan MJ, Maiofiss L, Cussac D, Audinot V, Boutin JA, Newman-Tancredi A (November 2002). "Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. I. A multivariate analysis of the binding profiles of 14 drugs at 21 native and cloned human receptor subtypes". J Pharmacol Exp Ther. 303 (2): 791–804. doi:10.1124/jpet.102.039867. PMID 12388666. S2CID 6200455.
- ↑ Newman-Tancredi A, Cussac D, Audinot V, Nicolas JP, De Ceuninck F, Boutin JA, Millan MJ (November 2002). "Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. II. Agonist and antagonist properties at subtypes of dopamine D(2)-like receptor and alpha(1)/alpha(2)-adrenoceptor". J Pharmacol Exp Ther. 303 (2): 805–14. doi:10.1124/jpet.102.039875. PMID 12388667. S2CID 35238120.
- ↑ Newman-Tancredi A, Cussac D, Quentric Y, Touzard M, Verrièle L, Carpentier N, Millan MJ (November 2002). "Differential actions of antiparkinson agents at multiple classes of monoaminergic receptor. III. Agonist and antagonist properties at serotonin, 5-HT(1) and 5-HT(2), receptor subtypes". J Pharmacol Exp Ther. 303 (2): 815–22. doi:10.1124/jpet.102.039883. PMID 12388668. S2CID 19260572.
- 1 2 "Archived copy". pdsp.unc.edu. Archived from the original on 13 April 2021. Retrieved 15 January 2022.
{{cite web}}: CS1 maint: archived copy as title (link) - ↑ Janssen W, Schymura Y, Novoyatleva T, Kojonazarov B, Boehm M, Wietelmann A, et al. (2015). "5-HT2B receptor antagonists inhibit fibrosis and protect from RV heart failure". BioMed Research International. 2015: 438403. doi:10.1155/2015/438403. PMC 4312574. PMID 25667920.
- ↑ Presseportal (Swiss press portal, in German)
- ↑ TheDay.com 5/10/2010