Angiomyolipoma
| Angiomyolipoma | |
|---|---|
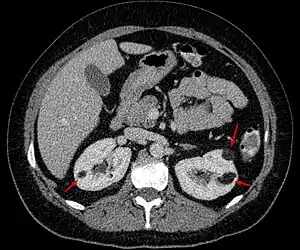 | |
| Angiomyolipoma in both kidneys (arrows) as seen on CT scan. The tumors are hypodense (dark) due to fat content. | |
| Specialty | Nephrology |
| Symptoms | None, blood in the urine, pain[1] |
| Complications | Hemorrhagic shock, kidney failure[2][3] |
| Usual onset | 20 to 50 years[3] |
| Causes | Tuberous sclerosis, lymphangioleiomyomatosis, spontaneous[1][3] |
| Diagnostic method | CT scan or MRI[3] |
| Differential diagnosis | Renal cell cancer[3] |
| Treatment | Surveillance, surgical removal, embolisation,ablation[3] |
| Frequency | Uncommon[3] |
Angiomyolipoma (AML) is a benign tumor of fat, blood vessels, and muscle tissue, that is usually found in the kidney.[1][2] They rarely cause symptoms, but may occasionally result in blood in the urine or grow to a size that pain occurs.[1][3] Complications can include hemorrhagic shock and kidney failure.[2][3] Occasionally other organs like the liver may be affected.[2]
They occur in up to 90% of cases of tuberous sclerosis (TSC) and 50% of cases of lymphangioleiomyomatosis (LAM).[1][3] In tuberous sclerosis often multiple lesions are present.[3] They may also occur spontaneously.[3] They can usually be diagnosed by CT scan or MRI.[3]
Small angiomyolipoma that do not result in symptoms can often we simple watched.[3] Larger or symptomatic cases may be treated by surgical removal, embolisation, or ablation.[3] In cases due to TSC or LAM, mTOR inhibitors may be an option.[3] Occasionally dialysis or kidney transplant is required.[2] They are uncommon.[3] Females are more commonly affected than males.[3] Onset is generally between 20 and 50 years of age.[3] It was first described in 1900.[3]
Signs and symptoms
If the dilated blood vessels in an angiomyolipoma rupture, the resulting retroperitoneal haemorrhage causes sudden pain, accompanied with nausea and vomiting. When the patient presents in the emergency department, up to 20% are in shock.[4]
Pathophysiology
Angiomyolipomas are tumours consisting of perivascular epithelioid cells (cells which are found surrounding blood vessels and which resemble epithelial cells). A tumour of this kind is known as a PEComa, from the initials of perivascular epithelioid cell. Older literature may classify them as hamartomas (benign tumours consisting of cells in their correct location, but forming a disorganised mass) or choristoma (benign tumours consisting of normal cells in the wrong location). PEComas are themselves a kind of mesenchymal tumour which involves cells that form the connective tissue, cardiovascular, and lymphatic systems.[4]
An angiomyolipoma is composed of varying proportions of vascular cells, immature smooth muscle cells, and fat cells.[4] These three components respectively give rise to the components of the name: angio-, myo-, and lip-. The -oma suffix indicates a tumour.
Angiomyolipomas are typically found in the kidney, but have also been commonly found in the liver and less commonly the ovary, fallopian tube, spermatic cord, palate, and colon. The Maclean imaging classification system for renal angiomyolipomas is based on the location of the angiomyolipoma within the kidney.[5]
Since all three components of an angiomyolipoma (vascular cells, immature smooth muscle cells, and fat cells) contain a "second-hit" mutation, they are believed to have derived from a common progenitor cell that suffered the common second-hit mutation.[4]
Diagnosis
Three methods of scanning can detect angiomyolipoma: ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI). Ultrasound is standard and is particularly sensitive to the fat in angiomyolipoma, but less so to the solid components. However, accurate measurements are hard to make with ultrasound, particularly if the angiomyolipoma is near the surface of the kidney (grade III).[5] CT is very detailed and fast, and allows accurate measurement. However, it exposes the patient to radiation and the dangers that a contrast dye used to aid the scanning may itself harm the kidneys. MRI is safer than CT, but many patients (particularly those with the learning difficulties or behavioural problems found in tuberous sclerosis) require sedation or general anaesthesia, and the scan cannot be performed quickly.[4] Some other kidney tumours contain fat, so the presence of fat is not diagnostic. Distinguishing a fat-poor angiomyolipoma from a renal cell carcinoma (RCC) can be difficult.[6] Both minimal fat AMLs and 80% of the clear-cell type of RCCs display signal drop on an out-of-phase MRI sequence compared to in-phase.[7] Thus, a lesion growing at greater than 5 mm per year may warrant a biopsy for diagnosis.[4]
Incidental discovery of angiomyolipomas should trigger consideration of tuberous sclerosis complex (TSC) and lymphangioleiomyomatosis, especially if they are large, bilateral, and/or multiple. Screening for TSC includes a detailed physical exam, including dermatologic and ophthalmologic evaluations, by TSC expert clinicians and a CT or MRI of the brain. Screening for LAM includes a high-resolution CT of the lung and pulmonary function testing.
 Angiomyolipoma seen as a hyperechoic mass in the upper pole of an adult kidney on renal ultrasonography.
Angiomyolipoma seen as a hyperechoic mass in the upper pole of an adult kidney on renal ultrasonography.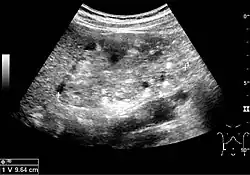 Renal ultrasonography of a person with tuberous sclerosis and multiple angiomyolipomas in the kidney: Measurement of kidney length on the US image is illustrated by ‘+’ and a dashed line.
Renal ultrasonography of a person with tuberous sclerosis and multiple angiomyolipomas in the kidney: Measurement of kidney length on the US image is illustrated by ‘+’ and a dashed line.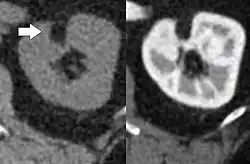 CT scan of a renal angiomyolipoma. It involves the renal cortex, and has an attenuation of less than 20 HU on the Hounsfield scale, which are typical characteristics.[8]
CT scan of a renal angiomyolipoma. It involves the renal cortex, and has an attenuation of less than 20 HU on the Hounsfield scale, which are typical characteristics.[8]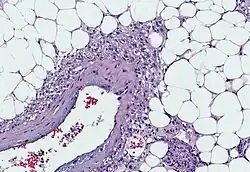 Myoid cells with clear cytoplasm spinning off of large vessels in a background of mature fat, the classic microscopic features of angiomyolipoma
Myoid cells with clear cytoplasm spinning off of large vessels in a background of mature fat, the classic microscopic features of angiomyolipoma
Treatment
Small angiomyolipoma that do not result in symptoms can often be simple watched.[3] Larger or symptomatic cases may be treated by surgical removal, embolisation, or ablation.[3] Occasionally dialysis or kidney transplant is required.[2]
Some centres may perform preventative selective embolisation of the angiomyolipoma if it is more than 4 cm in diameter, due to the risk of bleeding.[9]
People with tuberous sclerosis are advised to have yearly renal scans, though patients with very stable lesions could be monitored less frequently. The research in this area is lacking. Even if no angiomyolipoma is found, one can develop at any life stage. The angiomyolipoma can grow rapidly.[4]
In tuberous sclerosis, typically, many angiomyolipomas affect each kidney. Not uncommonly, more than one intervention may be required during lifetime. Since kidney function may already be impaired (up to half the kidney may be lost before function loss is detectable), preserving as much kidney as possible is vital when removing any lesion. Large angiomyolipomas are treated by embolization, which reduces the risk of haemorrhage and can also shrink the lesion. A side effect of this treatment is postembolisation syndrome, severe pain and fever, but this is easily managed and lasts only a few days.[4] Everolimus may be considered for asymptomatic, growing AMLs measuring larger than 3 cm in diameter.[2]
A ruptured aneurysm in an angiomyolipoma leads to blood loss that must be stopped (though embolisation) and compensated for (through intravenous fluid replacement). Therefore, removal of the affected kidney (nephrectomy) is strongly discouraged, though may occur if the emergency department is not knowledgeable about tuberous sclerosis.[4]
Embolisation involves inserting a catheter along the blood vessels to the tumour. The blood vessels are then blocked, typically by injecting ethanol or inert particles. The procedure can be very painful, so analgesics are used. The destroyed kidney tissue often causes postembolisation syndrome, which manifests as nausea, vomiting, fever, and abdominal pain, and lasts a few days. Embolisation (in general) has an 8% rate of morbidity and a 2.5% rate of mortality, so is not considered lightly.[9]
People with kidney loss should be monitored for hypertension (and treated for it if discovered) and avoid nephrotoxic drugs such as certain pain relievers and intravenous contrast agents. Such patients who are unable to communicate effectively (due to age or intellectual disability) are at risk of dehydration. Where multiple or large angiomyolipomas have caused chronic kidney disease, dialysis is required.[4]
Robotic assisted partial nephrectomy has been proposed as a surgical treatment of a ruptured angiomyolipoma combining the advantages both of a kidney preservation procedure and the benefits of a minimal invasive procedure without compromising the safety of the patient.[10]
Follow-up
It is suggested that no follow-up is needed for angiomyolipoma-like incidental imaging findings measuring less than 1 or 2 cm.[11][12] Alternatively, a renal ultrasonography every 3 or 4 years has been suggested for angiomyolipoma-like masses measuring less than 2 cm.[13] For those measuring 2 to 4 cm, annual ultrasonography is recommended.[13] Those over 4 cm are usually surgically removed, but for those that are not, it is recommended to perform an ultrasonography after 6 months, and then annually if stable.[13]
Prognosis
Small angiomyolipomas and those without dilated blood vessels (aneurysms) cause few problems, but angiomyolipomas have been known to grow as rapidly as 4 cm in one year. Angiomyolipomas larger than 5 cm and those containing an aneurysm pose a significant risk of rupture, which is a medical emergency, as it is potentially life-threatening. One population study found the cumulative risk of haemorrhage to be 10% in males and 20% in females.[4]
A second problem occurs when the renal angiomyolipomas take over so much kidney that the function is impaired, leading to chronic kidney disease. This may be severe enough to require dialysis. A population survey of patients with TSC and normal intelligence found 1% were on dialysis.[4]
Epidemiology
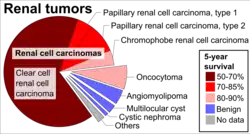
Angiomyolipomas are the most common benign tumour of the kidney, and are found either in patients with tuberous sclerosis or sporadically. About 80–90% of cases are sporadic, and these are most commonly found in middle-aged women.[6]
In patients with TSC, a longitudinal study found 80% will have some form of renal lesion by around 10 years of age. Of these, 75% are angiomyolipomas and 17% are cysts. The angiomyolipomas increased in size in around 60% of these children. An autopsy study and TSC clinic survey found a prevalence of 67 and 85%, respectively, for patients with TSC. Both genders are affected equally.[4]
References
- 1 2 3 4 5 "NCI Search Results - National Cancer Institute". www.cancer.gov. 2 February 2011. Archived from the original on 27 August 2021. Retrieved 26 January 2021.
- 1 2 3 4 5 6 7 Northrup, H; Krueger, DA (2013). "International Tuberous Sclerosis Complex Consensus Group. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference". Pediatr Neurol. 49 (4): 243–254. doi:10.1016/j.pediatrneurol.2013.08.001. PMC 4080684. PMID 24053982.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 Flum, AS; Hamoui, N; Said, MA; Yang, XJ; Casalino, DD; McGuire, BB; Perry, KT; Nadler, RB (April 2016). "Update on the Diagnosis and Management of Renal Angiomyolipoma". The Journal of urology. 195 (4 Pt 1): 834–46. doi:10.1016/j.juro.2015.07.126. PMID 26612197.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Bissler JJ, Henske EP. Renal Manifestations of Tuberous Sclerosis Complex. In: Kwiatkowski DJ, Wiittlemore DJ, Thiele EA, editors. Tuberous Sclerosis Complex: Genes, Clinical Features and Therapeutics. Wiley-VCH Verlag GmbH; 2010. p. 321–325. ISBN 3-527-32201-9.
- 1 2 D. F. W. Maclean, R. Sultana, R. Radwan, L. McKnight, and J. Khastgir, Is the follow-up of small renal angiomyolipomas a necessary precaution? Archived 2016-12-31 at the Wayback Machine Clinical Radiology, vol.69, no.8, pp.822–826, 2014
- 1 2 Shin, NY; Kim, MJ; Chung, JJ; Chung, YE; Choi, JY; Park, YN (May–Jun 2010). "The differential imaging features of fat-containing tumors in the peritoneal cavity and retroperitoneum: the radiologic-pathologic correlation" (PDF). Korean Journal of Radiology. 11 (3): 333–45. doi:10.3348/kjr.2010.11.3.333. PMC 2864861. PMID 20461188. Archived (PDF) from the original on 2021-08-27. Retrieved 2010-09-03.
- ↑ Rinze Reinhard, Mandy van der Zon-Conijn and Robin Smithuis. "Kidney - Solid masses". Radiology Assistant. Archived from the original on 2017-10-27. Retrieved 2017-10-27.
- ↑ Dr Yuranga Weerakkody and Dr Behrang Amini. "Renal angiomyolipoma". Radiopaedia. Archived from the original on 2019-07-11. Retrieved 2019-08-02.
- 1 2 Loffroy, R; Rao, P; Kwak, BK; Ota, S; De Lin, M; Liapi, E; Geschwind, JF (May–Jun 2010). "Transcatheter arterial embolization in patients with kidney diseases: an overview of the technical aspects and clinical indications" (PDF). Korean Journal of Radiology. 11 (3): 257–68. doi:10.3348/kjr.2010.11.3.257. PMC 2864852. PMID 20461179. Archived (PDF) from the original on 2021-08-27. Retrieved 2010-09-06.
- ↑ Ploumidis, A; Katafigiotis, I; Thanou, M; Bodozoglou, N; Athanasiou, L; Ploumidis, A (2013). "Spontaneous Retroperitoneal Hemorrhage (Wunderlich Syndrome) due to Large Upper Pole Renal Angiomyolipoma: Does Robotic-Assisted Laparoscopic Partial Nephrectomy Have a Role in Primary Treatment?". Case Reports in Urology. 2013: 498694. doi:10.1155/2013/498694. PMC 3784227. PMID 24106637.
- ↑ Doshi, Ankur M.; Ayoola, Abimbola; Rosenkrantz, Andrew B. (2017). "Do Incidental Hyperechoic Renal Lesions Measuring Up to 1 cm Warrant Further Imaging? Outcomes of 161 Lesions". American Journal of Roentgenology. 209 (2): 346–350. doi:10.2214/AJR.16.17490. ISSN 0361-803X.
- ↑ Maclean, D.F.W.; Sultana, R.; Radwan, R.; McKnight, L.; Khastgir, J. (2014). "Is the follow-up of small renal angiomyolipomas a necessary precaution?". Clinical Radiology. 69 (8): 822–826. doi:10.1016/j.crad.2014.03.016. ISSN 0009-9260.
- 1 2 3 Vicente E Torres, York Pei. "UpToDate". UpToDate. Archived from the original on 2019-05-15. Retrieved 2019-05-15. This topic last updated: Dec 30, 2017.
External links
| Classification |
|---|