Congenital muscular dystrophy
| Congenital muscular dystrophy | |
|---|---|
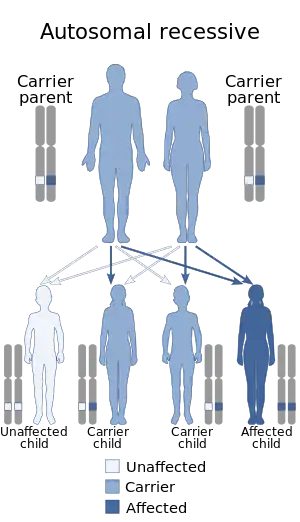 | |
| Autosomal recessive is generally the manner in which CMD is inherited | |
| Symptoms | Muscle weakness[1] |
| Types | 17 types of CMD[1] |
| Diagnostic method | NRI, EMG[2] |
| Treatment | Currently there's no cure; one should monitor cardiac function and respiratory function[3] |
Congenital muscular dystrophies are autosomal recessively-inherited muscle diseases. They are a group of heterogeneous disorders characterized by muscle weakness which is present at birth and the different changes on muscle biopsy that ranges from myopathic to overtly dystrophic due to the age at which the biopsy takes place.[1][4]
Signs and symptoms
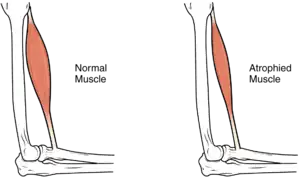
Most infants with CMD will display some progressive muscle weakness or muscle wasting (atrophy), although there can be different degrees and symptoms of severeness of progression. The weakness is indicated as hypotonia, or lack of muscle tone, which can make an infant seem unstable.[1][5]
Children may be slow with their motor skills; such as rolling over, sitting up or walking, or may not even reach these milestones of life. Some of the rarer forms of CMD can result in significant learning disabilities.
Genetics
The genetics of congenital muscular dystrophy are autosomal recessive which means two copies of an abnormal gene must be present for the disease or trait to happen. In the case of collagen VI-deficient, it is autosomal dominant, which means a child could inherit the disease from only one copy of a gene present in only one parent.[1]
The prevalence for congenital muscular dystrophy seems to be between 2.6 and 4.5 in 10,000 according to Reed, 2009.[6] MDCIA, for example is due to a mutation in the LAMA-2 gene and is involved with the 6q2 chromosome.[7]
Mechanism
In terms of the mechanism of congenital muscular dystrophy, one finds that though there are many types of CMD the glycosylation of α-dystroglycan and alterations in those genes that are involved are an important part of this conditions pathophysiology[8]
Diagnosis
For the diagnosis of congenital muscular dystrophy, the following tests/exams are done:[2]
- Lab study (CK levels)
- Muscle MRI and especially whole body muscle MRI has recently been used to describe muscle abnormalities in patients with primary laminin-α2 (merosin) deficiency subtype of CMD.
- EMG
- Genetic testing
Classification (different types of congenital muscular dystrophies)
The subtypes of congenital muscular dystrophy have been established through variations in multiple genes. Phenotype, as well as, genotype classifications are used to establish the subtypes, in some literature.[1]
One finds that congenital muscular dystrophies can be either autosomal dominant or autosomal recessive in terms of the inheritance pattern, though the latter is much more common[1]
Individuals who suffer from congenital muscular dystrophy fall into one of the following types:
- CMD with brain-eye, also called muscle-eye-brain disease,[9] is a rare form of congenital muscular dystrophy (autosomal recessive disorder) causing a lack of normal muscle tone which can delay walking due to being weak, also paralysis of eye muscles and intellectual disability which affects an individuals way of processing information[9] It is caused by a mutation in the POMGNT1 gene.[9]
- CMD with adducted (drawn inward) thumbs. a rare form of CMD causing permanent shortening of the toe joints and lack of muscle tone which can delay walking due to the individual being weak. The person with this form of congenital muscular dystrophy might have mild cerebellar hypoplasia in some cases .[1]
- CMD/LGMD without MR-first years of a newborn begins with weakness, which affects motive skills, walking can be accomplished in adolescence, deformity and rigidity of joints. The joints, neck and spine; progressive cardiomyopathy at the early ages; cardiac rhythm abnormalities may be present in the individual.[1]
- Large related CMD at the beginning of the newborn period, the issues the infant receives are; poor muscle tone and weak motor function; the individual will present with intellectual disability and the structure of the brain will likely be abnormal .[10]
- CMD with cerebellar atrophy severe cerebellar hypoplasia, poor muscle tone, delayed in motor milestones, lack of coordination in motive skills, difficulty speaking, involuntary movements and some intellectual disability. Furthermore, muscle biopsy does not reveal any deficiency.[1]
- Walker–Warburg syndrome at the beginning a progressive weakness and low muscle tone at birth or during early infancy; small muscles; the majority of affected children do not live more than 3 years of age. Eye structure problems are present, with accompanying visual impairment.[11]
- CMD with primary laminin-α2 (merosin) deficiency (MDC1A) intellect in such individuals is unaffected, proximal muscle weakening and rigid spine are present along with respiratory involvement (with disease progression).[12]
- CMD/LGMD with MR weakness and deformity and rigidity joints present at birth, poor muscle tone, slowly progressive; individuals may present with cerebellar cysts (or cortical problems), microcephaly may be present as well. Abnormal flexibility might occur, spinal curvature possible.[1]
- CDG I (DPM3) some of the symptoms at birth and throughout the infant's life are weakness or poor muscle tone. The individual may present with cardiomyopathy (no outflow obstruction), a rise in serum creatine kinase might be present as well. Some IQ problems may be present, along with weakness in the proximal muscles. Also of note, a reduction of dolichol phosphate mannose .[13]
- CDG I (DPM2) weak muscle tone starting in first weeks of the infant, the individual may show severe neurologic physical characteristics that result in fatality early in life. Hypotonia and myopathic facies may be present in such individuals, while contractures of joints may also be present. Finally, myoclonic seizures may occur at a very early age (3 months).[14]
- CDG Ie (DPM1) at birth the infant will have weakness with involvement of the respiratory system, as well as, severe mental and psychomotor problems.By age of 3, the individual may be blind with speech problems. Microcephaly may occur in early childhood, as well as seizures.[15]
- CMD with spinal rigidity present at birth can have poor muscle tone and weakness, reduced respiratory capacity, muscles could be deformed, beginning early ages stabilization or slow decline spinal rigidity, limited mobility to flex the neck and spine, spinal curvature and progressing deformity and rigidity joints, minor cardiac abnormalities, normal intelligence.[16]

- CMD with lamin A/C abnormality with in the first year the infant is weak, individual may have problems later lifting arms and head. May need nasogastric tube, limb weakness and elevated serum creatine kinase. Individual may show a diaphragmatic manner when breathing.[17]
- Integrin α7 weakness which is present at birth, poor muscle tone with late walking, loss of muscle tissue, intellectual disability.Furthermore, the creatine kinase level was elevated.[18]
- Fukuyama CMD-in Western countries this type of CMD is rare, but it is common in Japan. The effects this disease has on infants are on a spectrum of severity. They include weakness in muscle tone within the first year, deformed and rigid joints, spinal curvatures, seizures, eye involvement and intellectual disability. Some patients may achieve limited walking mobility.[19]
- Merosin-deficient CMD- weakness in muscle tone present at birth, spectrum of severity; may show hypotonia and poor motor development. Most individuals have periventricular white matter problems. However, intellectual disability is rare in most cases.[20]
- Merosin-positive CMD some forms of merosin-positive CMD are: Early spinal rigidity, CMD with muscle hypertrophy, CMD with muscle hypertrophy and respiratory failure.[21]
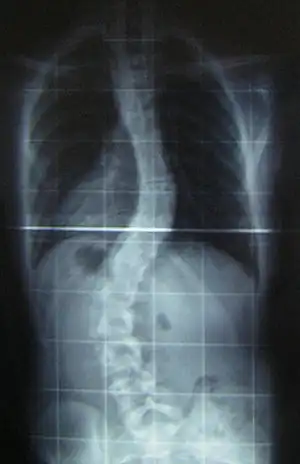
- Ullrich congenital muscular dystrophy present at birth is weakness, and poor muscle tone.
- Will have some deformity and rigidity joints, some joints will have excessive flexibility, spinal rigidity, curvature, respiratory impairment, soft skin, normal cardiac function and normal intelligence.[22]
Differential diagnosis
The DDx of congenital muscular dystrophy, in an affected individual, is as follows (non-neuromuscular genetic conditions also exist[23]):[2]
- Metabolic myopathies
- Dystrophinopathies
- Emery-Dreifuss muscular dystrophy
Management
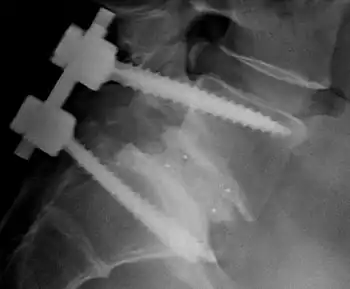
In terms of the management of congenital muscular dystrophy the American Academy of Neurology recommends that the individuals need to have monitoring of cardiac function, respiratory, and gastrointestinal. Additionally it is believed that therapy in speech, orthopedic and physical areas, would improve the person's quality of life.[3]
While there is currently no cure available, it is important to preserve muscle activity and any available correction of skeletal abnormalities (as scoliosis). Orthopedic procedures, like spinal fusion, maintains/increases the individual's prospect for more physical movement.[3]
See also
References
- 1 2 3 4 5 6 7 8 9 10 11 Sparks, Susan; Quijano-Roy, Susana; Harper, Amy; Rutkowski, Anne; Gordon, Erynn; Hoffman, Eric P.; Pegoraro, Elena (1993-01-01). "Congenital Muscular Dystrophy Overview – RETIRED CHAPTER, FOR HISTORICAL REFERENCE ONLY". In Pagon, Roberta A.; Adam, Margaret P.; Ardinger, Holly H.; Wallace, Stephanie E.; Amemiya, Anne; Bean, Lora J.H.; Bird, Thomas D.; Fong, Chin-To; Mefford, Heather C. (eds.). Congenital Muscular Dystrophy Overview. Seattle (WA): University of Washington, Seattle. PMID 20301468. Archived from the original on 2020-08-04. Retrieved 2021-10-14.update 2012
- 1 2 3 "Congenital Muscular Dystrophy Workup: Laboratory Studies, Imaging Studies, Other Tests". emedicine.medscape.com. Archived from the original on 2021-01-19. Retrieved 2016-04-28.
- 1 2 3 "Congenital muscular dystrophy". Guidelines American Academy of Neurology. 2015. Archived from the original on 19 October 2020. Retrieved 28 April 2016.
- ↑ Bertini, Enrico; D'Amico, Adele; Gualandi, Francesca; Petrini, Stefania (2011-12-01). "Congenital Muscular Dystrophies: A Brief Review". Seminars in Pediatric Neurology. 18 (4): 277–288. doi:10.1016/j.spen.2011.10.010. ISSN 1071-9091. PMC 3332154. PMID 22172424.
- ↑ "Hypotonia: MedlinePlus Medical Encyclopedia". www.nlm.nih.gov. Archived from the original on 2016-07-05. Retrieved 2016-04-28.
- ↑ Reed, Umbertina Conti (2009). "Congenital muscular dystrophy. Part I: a review of phenotypical and diagnostic aspects". Arquivos de Neuro-Psiquiatria. 67 (1): 144–168. doi:10.1590/S0004-282X2009000100038. ISSN 0004-282X. PMID 19330236.
- ↑ Reed, Umbertina (2009). "Congenital muscular dystrophy part 2" (PDF). Neuropsiquitria. Archived (PDF) from the original on 20 August 2017. Retrieved 28 April 2016.
- ↑ Martin, Paul T (2006). "Mechanisms of Disease: congenital muscular dystrophies—glycosylation takes center stage". Nature Clinical Practice Neurology. 2 (4): 222–230. doi:10.1038/ncpneuro0155. ISSN 1745-834X. PMC 2855642. PMID 16932553.
- 1 2 3 "OMIM Entry - # 253280 - MUSCULAR DYSTROPHY-DYSTROGLYCANOPATHY (CONGENITAL WITH BRAIN AND EYE ANOMALIES), TYPE A, 3; MDDGA3". www.omim.org. Archived from the original on 2015-12-11. Retrieved 2016-04-26.
- ↑ "Error 403".
- ↑ Reference, Genetics Home. "Walker-Warburg syndrome". Genetics Home Reference. Archived from the original on 2018-07-28. Retrieved 2016-04-26.
- ↑ Quijano-Roy, Susana; Sparks, Susan; Rutkowski, Anne (1993-01-01). "LAMA2 Muscular Dystrophy". In Pagon, Roberta A.; Adam, Margaret P.; Ardinger, Holly H.; Wallace, Stephanie E.; Amemiya, Anne; Bean, Lora J.H.; Bird, Thomas D.; Fong, Chin-To; Mefford, Heather C. (eds.). LAMA2-Related Muscular Dystrophy. Seattle (WA): University of Washington, Seattle. PMID 22675738.update 2012
- ↑ "OMIM Entry - # 612937 - CONGENITAL DISORDER OF GLYCOSYLATION, TYPE Io; CDG1O". www.omim.org. Archived from the original on 2019-06-08. Retrieved 2016-04-26.
- ↑ "OMIM Entry - # 615042 - CONGENITAL DISORDER OF GLYCOSYLATION, TYPE Iu; CDG1U". www.omim.org. Archived from the original on 2019-06-23. Retrieved 2016-04-26.
- ↑ "OMIM Entry - # 608799 - CONGENITAL DISORDER OF GLYCOSYLATION, TYPE Ie; CDG1E". www.omim.org. Archived from the original on 2019-09-29. Retrieved 2016-04-26.
- ↑ "OMIM Entry - # 602771 - RIGID SPINE MUSCULAR DYSTROPHY 1; RSMD1". www.omim.org. Archived from the original on 2015-12-07. Retrieved 2016-04-26.
- ↑ "OMIM Entry - # 613205 - MUSCULAR DYSTROPHY, CONGENITAL, LMNA-RELATED". www.omim.org. Archived from the original on 2019-09-18. Retrieved 2016-04-26.
- ↑ "OMIM Entry - # 613204 - MUSCULAR DYSTROPHY, CONGENITAL, DUE TO INTEGRIN ALPHA-7 DEFICIENCY". www.omim.org. Archived from the original on 2021-12-15. Retrieved 2016-04-26.
- ↑ Reference, Genetics Home. "Fukuyama congenital muscular dystrophy". Genetics Home Reference. Archived from the original on 2016-05-13. Retrieved 2016-04-26.
- ↑ "OMIM Entry - # 607855 - MUSCULAR DYSTROPHY, CONGENITAL MEROSIN-DEFICIENT, 1A; MDC1A". www.omim.org. Archived from the original on 2020-04-14. Retrieved 2016-04-26.
- ↑ "OMIM Entry - % 609456 - MUSCULAR DYSTROPHY, CONGENITAL, MEROSIN-POSITIVE". www.omim.org. Archived from the original on 2021-12-15. Retrieved 2016-04-26.
- ↑ "OMIM Entry - # 254090 - ULLRICH CONGENITAL MUSCULAR DYSTROPHY 1; UCMD1". omim.org. Archived from the original on 2015-12-24. Retrieved 2016-04-26.
- ↑ Bönnemann, Carsten G.; Wang, Ching H.; Quijano-Roy, Susana; Deconinck, Nicolas; Bertini, Enrico; Ferreiro, Ana; Muntoni, Francesco; Sewry, Caroline; Béroud, Christophe; Mathews, Katherine D.; Moore, Steven A.; Bellini, Jonathan; Rutkowski, Anne; North, Kathryn N. (1 April 2014). "Diagnostic approach to the congenital muscular dystrophies". Neuromuscular Disorders. 24 (4): 289–311. doi:10.1016/j.nmd.2013.12.011. PMC 5258110. PMID 24581957.
Further reading
- A, Graziano; F, Bianco; A, D'Amico; I, Moroni; S, Messina; C, Bruno; E, Pegoraro; M, Mora; G, Astrea (2015-03-01). "Prevalence of congenital muscular dystrophy in Italy: a population study". Neurology. 84 (9): 904–911. doi:10.1212/WNL.0000000000001303. ISSN 0028-3878. PMC 4351663. PMID 25653289.
- Paco, Sonia; Casserras, Teresa; Rodríguez, Maria Angels; Jou, Cristina; Puigdelloses, Montserrat; Ortez, Carlos I.; Diaz-Manera, Jordi; Gallardo, Eduardo; Colomer, Jaume (2015-12-15). "Transcriptome Analysis of Ullrich Congenital Muscular Dystrophy Fibroblasts Reveals a Disease Extracellular Matrix Signature and Key Molecular Regulators". PLOS ONE. 10 (12): e0145107. Bibcode:2015PLoSO..1045107P. doi:10.1371/journal.pone.0145107. ISSN 1932-6203. PMC 4686057. PMID 26670220.
- Falsaperla, Raffaele; Praticò, Andrea D.; Ruggieri, Martino; Parano, Enrico; Rizzo, Renata; Corsello, Giovanni; Vitaliti, Giovanna; Pavone, Piero (31 August 2016). "Congenital muscular dystrophy: from muscle to brain". Italian Journal of Pediatrics. 42 (1): 78. doi:10.1186/s13052-016-0289-9. ISSN 1824-7288. PMC 5006267. PMID 27576556.
- "Summary of Evidence-based Guideline for PATIENTS and their FAMILIES CONGENITAL MUSCULAR DYSTROPHY". aaan.com. The American Academy of Neurology (AAN). Archived from the original on 19 October 2020. Retrieved 5 December 2017.
External links
| Classification | |
|---|---|
| External resources |