Contraceptive vaginal ring
| NuvaRing | |
|---|---|
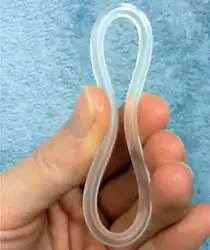 NuvaRing is a flexible plastic ring with both oestrogen and progesterone. | |
| Background | |
| Type | Hormonal |
| First use | 2001 |
| Failure rates (first year) | |
| Perfect use | 0.3 for Nuvaring%[1] |
| Typical use | 1.5 to 9 for Nuvaring%[2][1] |
| Usage | |
| Duration effect | 4 weeks for Nuvaring, 3 months for progesterone only vaginal ring |
| User reminders | Inserted for 3 weeks and then removed for 7 days for Nuvaring |
| Clinic review | Annual |
| Advantages and disadvantages | |
| STI protection | No |
| Weight | No proven effect |
| Benefits | Easy insertion and removal. |
A contraceptive vaginal ring is a type of hormonal insert that is placed in the vagina for the purpose of birth control. Some vaginal rings contain both an estrogen and a progestin (brand names NuvaRing and Annovera).[3][4][5] Other vaginal rings contain just progesterone (brand name Progering).[6]
Combined hormonal contraceptive vaginal ring
The ethinylestradiol/etonogestrel vaginal ring is also known as NuvaRing.[3] It is a flexible plastic (ethylene-vinyl acetate copolymer) ring that releases a low dose of a progestin and estrogen over three weeks.[7]
About 8% of women using a vaginal ring will still get pregnant every year, although this drops to 1% with perfect use. There is a small chance of blood clots, heart attacks and stroke with vaginal rings, and they are not recommended for women over 35 who smoke.[8]
The one-year combined hormonal contraceptive ring is also known as Annovera.[4] It is a silicone elastomer vaginal ring containing the progestin segesterone acetate and the estrogen ethinylestradiol.[9]
Progesterone only vaginal ring
A progesterone vaginal ring has also been developed.[2] It is specifically made for use during breast feeding as it does not affect milk production.[2]
References
- 1 2 Trussell, James (2011). "Contraceptive efficacy". In Hatcher, Robert A.; Trussell, James; Nelson, Anita L.; Cates, Willard Jr.; Kowal, Deborah; Policar, Michael S. (eds.). Contraceptive technology (20th revised ed.). New York: Ardent Media. pp. 779–863. ISBN 978-1-59708-004-0. ISSN 0091-9721. OCLC 781956734. Table 26–1 = Table 3–2 Percentage of women experiencing an unintended pregnancy during the first year of typical use and the first year of perfect use of contraception, and the percentage continuing use at the end of the first year. United States.
- 1 2 3 Birtley, Ellie J.; Lohr, Patricia A. (2014). "7. Emerging methods and methods not available in the United States". In Whitaker, Amy; Gilliam, Melissa (eds.). Contraception for Adolescent and Young Adult Women. Springer. p. 98–99. ISBN 9781461465799.
- 1 2 NUVARING- etonogestrel and ethinyl estradiol insert, extended release drug label/data at Daily Med from U.S. National Library of Medicine, National Institutes of Health.
- 1 2 ANNOVERA- segesterone acetate and ethinyl estradiol ring drug label/data at Daily Med from U.S. National Library of Medicine, National Institutes of Health.
- ↑ "Vaginal ring". nhs.uk. December 2017. Retrieved 25 August 2019.
- ↑ "Safety of the progesterone-releasing vaginal ring (PVR) among lactating women: A systematic review". Contraception. 94 (3): 253–261. 2016-09-01. doi:10.1016/j.contraception.2015.04.001. ISSN 0010-7824.
- ↑ "FSRH Clinical Guideline: Combined Hormonal Contraception (January 2019, Amended February 2019) - Faculty of Sexual and Reproductive Healthcare". www.fsrh.org. Retrieved 14 August 2019.
- ↑ "Vaginal Rings - Family Planning". www.familyplanning.org.nz. Retrieved 25 August 2019.
- ↑ "Prescribing Information: Annovera" (PDF). Drugs@FDA: FDA-Approved Drugs. August 2018. Retrieved 13 July 2020.