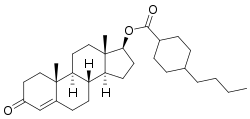
Testosterone buciclate
 | |
 | |
| Clinical data | |
|---|---|
| Other names | Testosterone bucyclate; Testosterone 17β-buciclate; 20 Aet-1; CDB-1781; Testosterone 17β-(trans-4-butylcyclohexyl)carboxylate |
| Routes of administration | Intramuscular injection |
| Drug class | Androgen; Anabolic steroid; Androgen ester |
| Pharmacokinetic data | |
| Bioavailability | Oral: very low Intramuscular: very high |
| Metabolism | Liver |
| Elimination half-life | Tea seed oil: 20.9 days (i.m.)[1][2] Castor oil: 33.9 days (i.m.)[1][2] |
| Excretion | Urine |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C30H46O3 |
| Molar mass | 454.695 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Testosterone buciclate (developmental code names 20 Aet-1, CDB-1781) is a synthetic, injected anabolic–androgenic steroid (AAS) which was never marketed.[3][4][5] It was developed in collaboration by the Contraceptive Development Branch (CDB) of the National Institute of Child Health and Human Development (NICHD) and the World Health Organization (WHO) in the 1970s and early 1980s for use in androgen replacement therapy for male hypogonadism and as a potential male contraceptive.[3] It was first described in 1986.[4] The medication is an androgen ester – specifically, the C17β buciclate (4-butylcyclohexane-1-carboxylate) ester of testosterone – and is a prodrug of testosterone with a very long duration of action when used as a depot via intramuscular injection.[3][6] Testosterone buciclate is formulated as a microcrystalline aqueous suspension with a defined particle size of at least 75% in the range of 10 to 50 μm.[7]
A single intramuscular injection of testosterone buciclate has been found to produce physiological levels of testosterone within the normal range in hypogonadal men for 3 to 4 months.[3][1][8][9][2] The elimination half-life and mean residence time (average amount of time a single molecule of drug stays in the body) of testosterone buciclate were found to be 29.5 days and 60.0 days, respectively, whereas those of testosterone enanthate in castor oil were only 4.5 days and 8.5 days.[8][9][2] Testosterone buciclate also lasts longer than testosterone undecanoate, which has elimination half-lives and mean residence times of 20.9 days and 34.9 days in tea seed oil and 33.9 days and 36.0 days in castor oil, respectively.[1][9][2] In addition, there is a spike in testosterone levels with testosterone enanthate and testosterone undecanoate that is not seen with testosterone buciclate, with which levels stay highly uniform and decrease very gradually and progressively.[1] Testosterone buciclate can maintain testosterone levels in the normal male range for up to 20 weeks with a single intramuscular injection.[10]
Testosterone buciclate is able to reversibly and completely suppress spermatogenesis in men when used at sufficiently high dosages.[8] As such, the results of clinical studies for use of testosterone buciclate as a male contraceptive were promising, and trials continued as late as 1995,[11] but progress ultimately came to a standstill because the WHO was unable to find an industry partner willing to continue the development of the drug.[1] Because of this, the WHO backed away from testosterone buciclate and focused its research instead on testosterone undecanoate, which is also very long-lasting and has the advantage of having already been marketed and approved for medical use.[12]
| Route | Medication | Major brand names | Form | Dosage |
|---|---|---|---|---|
| Oral | Testosteronea | – | Tablet | 400–800 mg/day (in divided doses) |
| Testosterone undecanoate | Andriol, Jatenzo | Capsule | 40–80 mg/2–4x day (with meals) | |
| Methyltestosteroneb | Android, Metandren, Testred | Tablet | 10–50 mg/day | |
| Fluoxymesteroneb | Halotestin, Ora-Testryl, Ultandren | Tablet | 5–20 mg/day | |
| Metandienoneb | Dianabol | Tablet | 5–15 mg/day | |
| Mesteroloneb | Proviron | Tablet | 25–150 mg/day | |
| Sublingual | Testosteroneb | Testoral | Tablet | 5–10 mg 1–4x/day |
| Methyltestosteroneb | Metandren, Oreton Methyl | Tablet | 10–30 mg/day | |
| Buccal | Testosterone | Striant | Tablet | 30 mg 2x/day |
| Methyltestosteroneb | Metandren, Oreton Methyl | Tablet | 5–25 mg/day | |
| Transdermal | Testosterone | AndroGel, Testim, TestoGel | Gel | 25–125 mg/day |
| Androderm, AndroPatch, TestoPatch | Non-scrotal patch | 2.5–15 mg/day | ||
| Testoderm | Scrotal patch | 4–6 mg/day | ||
| Axiron | Axillary solution | 30–120 mg/day | ||
| Androstanolone (DHT) | Andractim | Gel | 100–250 mg/day | |
| Rectal | Testosterone | Rektandron, Testosteronb | Suppository | 40 mg 2–3x/day |
| Injection (IM or SC) | Testosterone | Andronaq, Sterotate, Virosterone | Aqueous suspension | 10–50 mg 2–3x/week |
| Testosterone propionateb | Testoviron | Oil solution | 10–50 mg 2–3x/week | |
| Testosterone enanthate | Delatestryl | Oil solution | 50–250 mg 1x/1–4 weeks | |
| Xyosted | Auto-injector | 50–100 mg 1x/week | ||
| Testosterone cypionate | Depo-Testosterone | Oil solution | 50–250 mg 1x/1–4 weeks | |
| Testosterone isobutyrate | Agovirin Depot | Aqueous suspension | 50–100 mg 1x/1–2 weeks | |
| Testosterone phenylacetateb | Perandren, Androject | Oil solution | 50–200 mg 1x/3–5 weeks | |
| Mixed testosterone esters | Sustanon 100, Sustanon 250 | Oil solution | 50–250 mg 1x/2–4 weeks | |
| Testosterone undecanoate | Aveed, Nebido | Oil solution | 750–1,000 mg 1x/10–14 weeks | |
| Testosterone buciclatea | – | Aqueous suspension | 600–1,000 mg 1x/12–20 weeks | |
| Implant | Testosterone | Testopel | Pellet | 150–1,200 mg/3–6 months |
| Notes: Men produce about 3 to 11 mg testosterone per day (mean 7 mg/day in young men). Footnotes: a = Never marketed. b = No longer used and/or no longer marketed. Sources: See template. | ||||
| Testosterone ester | Form | Route | Tmax | t1/2 | MRT |
|---|---|---|---|---|---|
| Testosterone undecanoate | Oil-filled capsules | Oral | ? | 1.6 hours | 3.7 hours |
| Testosterone propionate | Oil solution | Intramuscular injection | ? | 0.8 days | 1.5 days |
| Testosterone enanthate | Castor oil solution | Intramuscular injection | 10 days | 4.5 days | 8.5 days |
| Testosterone undecanoate | Tea seed oil solution | Intramuscular injection | 13.0 days | 20.9 days | 34.9 days |
| Testosterone undecanoate | Castor oil solution | Intramuscular injection | 11.4 days | 33.9 days | 36.0 days |
| Testosterone buciclatea | Aqueous suspension | Intramuscular injection | 25.8 days | 29.5 days | 60.0 days |
| Notes: Testosterone cypionate has similar pharmacokinetics to TE. Footnotes: a = Never marketed. Sources: See template. | |||||
| Medication | Form | Major brand names | Duration |
|---|---|---|---|
| Testosterone | Aqueous suspension | Andronaq, Sterotate, Virosterone | 2–3 days |
| Testosterone propionate | Oil solution | Androteston, Perandren, Testoviron | 3–4 days |
| Testosterone phenylpropionate | Oil solution | Testolent | 8 days |
| Testosterone isobutyrate | Aqueous suspension | Agovirin Depot, Perandren M | 14 days |
| Mixed testosterone estersa | Oil solution | Triolandren | 10–20 days |
| Mixed testosterone estersb | Oil solution | Testosid Depot | 14–20 days |
| Testosterone enanthate | Oil solution | Delatestryl | 14–28 days |
| Testosterone cypionate | Oil solution | Depovirin | 14–28 days |
| Mixed testosterone estersc | Oil solution | Sustanon 250 | 28 days |
| Testosterone undecanoate | Oil solution | Aveed, Nebido | 100 days |
| Testosterone buciclated | Aqueous suspension | 20 Aet-1, CDB-1781e | 90–120 days |
| Nandrolone phenylpropionate | Oil solution | Durabolin | 10 days |
| Nandrolone decanoate | Oil solution | Deca Durabolin | 21–28 days |
| Methandriol | Aqueous suspension | Notandron, Protandren | 8 days |
| Methandriol bisenanthoyl acetate | Oil solution | Notandron Depot | 16 days |
| Metenolone acetate | Oil solution | Primobolan | 3 days |
| Metenolone enanthate | Oil solution | Primobolan Depot | 14 days |
| Note: All are via i.m. injection. Footnotes: a = TP, TV, and TUe. b = TP and TKL. c = TP, TPP, TiCa, and TD. d = Studied but never marketed. e = Developmental code names. Sources: See template. | |||
| Androgen | Structure | Ester | Relative mol. weight | Relative T contentb | logPc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position(s) | Moiet(ies) | Type | Lengtha | ||||||
| Testosterone | – | – | – | – | 1.00 | 1.00 | 3.0–3.4 | ||
| Testosterone propionate | C17β | Propanoic acid | Straight-chain fatty acid | 3 | 1.19 | 0.84 | 3.7–4.9 | ||
| Testosterone isobutyrate | C17β | Isobutyric acid | Aromatic fatty acid | – (~3) | 1.24 | 0.80 | 4.9–5.3 | ||
| Testosterone isocaproate | C17β | Isohexanoic acid | Branched-chain fatty acid | – (~5) | 1.34 | 0.75 | 4.4–6.3 | ||
| Testosterone caproate | C17β | Hexanoic acid | Straight-chain fatty acid | 6 | 1.35 | 0.75 | 5.8–6.5 | ||
| Testosterone phenylpropionate | C17β | Phenylpropanoic acid | Aromatic fatty acid | – (~6) | 1.46 | 0.69 | 5.8–6.5 | ||
| Testosterone cypionate | C17β | Cyclopentylpropanoic acid | Aromatic fatty acid | – (~6) | 1.43 | 0.70 | 5.1–7.0 | ||
| Testosterone enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.39 | 0.72 | 3.6–7.0 | ||
| Testosterone decanoate | C17β | Decanoic acid | Straight-chain fatty acid | 10 | 1.53 | 0.65 | 6.3–8.6 | ||
| Testosterone undecanoate | C17β | Undecanoic acid | Straight-chain fatty acid | 11 | 1.58 | 0.63 | 6.7–9.2 | ||
| Testosterone buciclated | C17β | Bucyclic acide | Aromatic carboxylic acid | – (~9) | 1.58 | 0.63 | 7.9–8.5 | ||
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic fatty acids. b = Relative testosterone content by weight (i.e., relative androgenic/anabolic potency). c = Experimental or predicted octanol/water partition coefficient (i.e., lipophilicity/hydrophobicity). Retrieved from PubChem, ChemSpider, and DrugBank. d = Never marketed. e = Bucyclic acid = trans-4-Butylcyclohexane-1-carboxylic acid. Sources: See individual articles. | |||||||||
See also
References
- 1 2 3 4 5 6 Eberhard Nieschlag; Hermann Behre (29 June 2013). Andrology: Male Reproductive Health and Dysfunction. Springer Science & Business Media. pp. 316, 412. ISBN 978-3-662-04491-9.
- 1 2 3 4 5 Behre HM, Abshagen K, Oettel M, Hübler D, Nieschlag E (1999). "Intramuscular injection of testosterone undecanoate for the treatment of male hypogonadism: phase I studies". Eur. J. Endocrinol. 140 (5): 414–9. CiteSeerX 10.1.1.503.1752. doi:10.1530/eje.0.1400414. PMID 10229906.
- 1 2 3 4 C. Coutifaris; L. Mastroianni (15 August 1997). New Horizons in Reproductive Medicine. CRC Press. pp. 100–. ISBN 978-1-85070-793-6.
- 1 2 William Llewellyn (2009). Anabolics. Molecular Nutrition Llc. pp. 138–140. ISBN 978-0967930473.
- ↑ Behre HM, Nieschlag E (1992). "Testosterone buciclate (20 Aet-1) in hypogonadal men: pharmacokinetics and pharmacodynamics of the new long-acting androgen ester". J. Clin. Endocrinol. Metab. 75 (5): 1204–10. doi:10.1210/jcem.75.5.1430080. PMID 1430080.
- ↑ Shalender Bhasin (13 February 1996). Pharmacology, Biology, and Clinical Applications of Androgens: Current Status and Future Prospects. John Wiley & Sons. pp. 472–. ISBN 978-0-471-13320-9.
- ↑ Hermann M. Behre; Gerhard F. Weinbauer; Eberhard Nieschlag (13 February 1996). "Testosterone Buciclate". In Shalender Bhasin; Henry L. Gabelnick; Jeffrey M. Spieler (eds.). Pharmacology, Biology, and Clinical Applications of Androgens: Current Status and Future Prospects. John Wiley & Sons. pp. 471–480. ISBN 978-0-471-13320-9.
Testosterone buciclate is applied intramuscularly as a microcrystalline aqueous suspension. [...] After air milling [...] of crystalline testosterone buciclate to a particle size of at least 75% in the range of 10 - 50 μm, the drug was [...] suspended in sterile, aqueous suspension vehicle [...].
- 1 2 3 Anita H. Payne; Matthew P. Hardy (28 October 2007). The Leydig Cell in Health and Disease. Springer Science & Business Media. pp. 423–. ISBN 978-1-59745-453-7.
- 1 2 3 Eberhard Nieschlag; Hermann M. Behre; Susan Nieschlag (13 January 2010). Andrology: Male Reproductive Health and Dysfunction. Springer Science & Business Media. pp. 441–446. ISBN 978-3-540-78355-8.
- ↑ Carrie Bagatell; William J. Bremner (27 May 2003). Androgens in Health and Disease. Springer Science & Business Media. pp. 146–. ISBN 978-1-59259-388-0.
- ↑ Behre HM, Baus S, Kliesch S, Keck C, Simoni M, Nieschlag E (1995). "Potential of testosterone buciclate for male contraception: endocrine differences between responders and nonresponders". J. Clin. Endocrinol. Metab. 80 (8): 2394–403. doi:10.1210/jcem.80.8.7543113. PMID 7543113.
- ↑ Nieschlag E, Kumar N, Sitruk-Ware R (2013). "7α-methyl-19-nortestosterone (MENTR): the population council's contribution to research on male contraception and treatment of hypogonadism". Contraception. 87 (3): 288–95. doi:10.1016/j.contraception.2012.08.036. PMID 23063338.