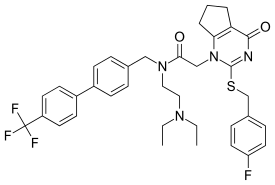
Darapladib
 | |
| Clinical data | |
|---|---|
| Other names | SB-480848 |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.130.738 |
| Chemical and physical data | |
| Formula | C36H38F4N4O2S |
| Molar mass | 666.78 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Darapladib is an inhibitor lipoprotein-associated phospholipase A2 (Lp-PLA2) that is in development as a drug for treatment of atherosclerosis.[1]
It was discovered by Human Genome Sciences in collaboration with GlaxoSmithKline (GSK).[2]
In November 2013, GSK announced that the drug had failed to meet Phase III endpoints in a trial of 16,000 patients with acute coronary syndrome (ACS).[3] An additional trial of 13,000 patients (SOLID-TIMI 52) finished in May 2014. The study failed to reduce the risk of coronary heart disease death, myocardial infarction, and urgent coronary revascularization compared with placebo in acute coronary syndrome patients treated with standard medical care.[4]
References
- ↑ Thompson PL, Nidorf SM, Eikelboom J (August 2013). "Targeting the unstable plaque in acute coronary syndromes". Clinical Therapeutics. 35 (8): 1099–107. doi:10.1016/j.clinthera.2013.07.332. PMID 23973042.
- ↑ "Spotlight on Glaxo Heart Drug as Others Fail". Reuters. 12 April 2007.
- ↑ Carroll J (12 November 2013). "GlaxoSmithKline loses its first big PhIII bet on heart drug darapladib". Fierce Biotech.
- ↑ "GSK announces phase III study with darapladib did not meet primary endpoint in patients following an acute coronary syndrome". GlaxoSmithKline plc. 13 May 2014. Archived from the original on 14 July 2014. Retrieved 2 July 2014.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.