Acute coronary syndrome
| Acute coronary syndrome | |
|---|---|
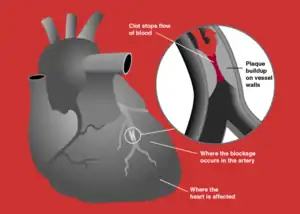 | |
| Blockage of a coronary artery | |
| Specialty | Cardiology |
Acute coronary syndrome (ACS) is a syndrome (a set of signs and symptoms) due to decreased blood flow in the coronary arteries such that part of the heart muscle is unable to function properly or dies.[1] The most common symptom is centrally located pressure-like chest pain, often radiating to the left shoulder[2] or angle of the jaw, and associated with nausea and sweating. Many people with acute coronary syndromes present with symptoms other than chest pain, particularly women, older people, and people with diabetes mellitus.[3]
Acute coronary syndrome is subdivided in three scenarios depending on the duration of symptoms, the presence of ECG changes and blood test results:[4] ST elevation myocardial infarction (STEMI, 30%), non-ST elevation myocardial infarction (NSTEMI, 25%), or unstable angina (38%).[5] Generally, when symptoms occur for less than 30 minutes, it is unstable angina. When symptoms are prolonged for more than 30 minutes, the diagnosis is acute myocardial infarction.[6]
ACS should be distinguished from stable angina, which develops during physical activity or stress and resolves at rest. In contrast with stable angina, unstable angina occurs suddenly, often at rest or with minimal exertion, or at lesser degrees of exertion than the individual's previous angina ("crescendo angina"). New-onset angina is also considered unstable angina, since it suggests a new problem in a coronary artery.
Signs and symptoms
The cardinal symptom of critically decreased blood flow to the heart is chest pain, experienced as tightness around or over the chest and (often, but not always) radiating to the left arm and the left angle of the jaw. This may be associated with diaphoresis (sweating), nausea and vomiting, as well as shortness of breath. In many cases, the sensation is "atypical", with pain experienced in different ways or even being completely absent (which is more likely in female patients and those with diabetes). Some may report palpitations, anxiety or a sense of impending doom (angor animi) and a feeling of being acutely ill. The description of the chest discomfort as a pressure has little utility in aiding a diagnosis as it is not specific for ACS.[7]
Though ACS is usually associated with coronary thrombosis, it can also be associated with cocaine use.[8] Chest pain with features characteristic of cardiac origin (angina) can also be precipitated by profound anemia, brady- or tachycardia (excessively slow or rapid heart rate), low or high blood pressure, severe aortic valve stenosis (narrowing of the valve at the beginning of the aorta), pulmonary artery hypertension and a number of other conditions.[9]
Pathophysiology

In those who have ACS, atheroma rupture is most commonly found 60% when compared to atheroma erosion (30%), thus causes the formation of thrombus which block the coronary arteries. Plaque rupture is responsible for 60% in ST elevated myocardial infarction (STEMI) while plaque erosion is responsible for 30% of the STEMI and vice versa for Non ST elevated myocardial infarction (NSTEMI). In plaque rupture, the content of the plaque are lipid rich, collagen poor, with abundant inflammation which is macrophage predominant, and covered with a thin fibrous cap. Meanwhile, in plaque erosion, the plaque is rich with extracellular matrix, proteoglycan, glycoaminoglycan, but without fibrous caps, no inflammatory cells, and no large lipid core. After the coronary arteries are unblocked, there is a risk of reperfusion injury due spreading inflammatory mediators throughout the body. Investigations is still underway on the role of Cyclophilin D in reducing the reperfusion injury.[11]
Other, less common, causes of acute coronary syndrome include spontaneous coronary artery dissection [12] and myocardial infarction in the absence of obstructive coronary artery disease (MINOCA).[13]
Diagnosis
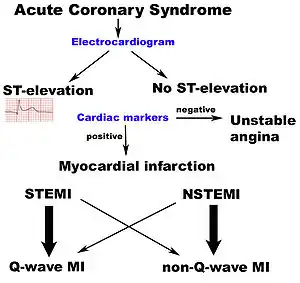
Electrocardiogram
In the setting of acute chest pain, the electrocardiogram is the investigation that most reliably distinguishes between various causes.[15] The ECG should be done as early as practicable, including in the ambulance if possible.[16] If this indicates acute heart damage (elevation in the ST segment, new left bundle branch block), treatment for a heart attack in the form of angioplasty or thrombolysis is indicated immediately (see below). In the absence of such changes, it is not possible to immediately distinguish between unstable angina and NSTEMI.
Imaging and blood tests
As it is only one of the many potential causes of chest pain, the patient usually has a number of tests in the emergency department, such as a chest X-ray, blood tests (including myocardial markers such as troponin I or T, and H-FABP and/or a D-dimer if a pulmonary embolism is suspected), and telemetry (monitoring of the heart rhythm).
Combination of troponin levels (less than 5 ng/L) with low TIMI scores can help to predict those with low possibility of myocardial infarction and discharge them safely from the emergency department.[11] Coronary CT angiography combined with Troponin levels is also helpful to triage those who are susceptible to ACS. F-fluoride positron emission tomography is also helpful in identifying those with high risk, lipid-rich coronary plaques.[11]
Prediction scores
The ACI-TIPI score can be used to aid diagnosis; using seven variables from the admission record, this score predicts crudely which patients are likely to have myocardial ischemia.[17] For example, according to a randomized controlled trial, males having chest pain with normal or non-diagnostic ECG are at higher risk for having acute coronary syndrome than women.[18] In this study, the sensitivity was 65.2% and specificity was 44%. This particular study had an 8.4% prevalence of acute coronary syndrome, which means the positive predictive value of being a male with chest pain and having coronary syndrome is 9.6% and negative predictive value is 93.2% ( click here to adjust these results for people at higher or lower risk of acute coronary syndrome). In a second cohort study, exercise electrocardiography was similarly found to be a poor predictor of acute coronary syndrome at follow-up.[19] Of the patients who had a coronary event at 6 years of follow up, 47% had a negative ECG at the start of the study. With an average follow up of 2.21 years the receiver operating characteristic curves gave resting ECG a score of 0.72 and exercise ECG a score of 0.74.
There are not only prediction scores for diagnosis of ACS, but also prognosis. Most notably, the GRACE ACS Risk and Mortality score helps diagnose, and based upon that score predicts mortality rate of a given patient. It takes into account both clinical (blood pressure, heart rate, EKG findings) and medical history in its scoring system.[20]
Prevention
Acute coronary syndrome often reflects a degree of damage to the coronaries by atherosclerosis. Primary prevention of atherosclerosis is controlling the risk factors: healthy eating, exercise, treatment for hypertension and diabetes, avoiding smoking and controlling cholesterol levels; in patients with significant risk factors, aspirin has been shown to reduce the risk of cardiovascular events. Secondary prevention is discussed in myocardial infarction.
After a ban on smoking in all enclosed public places was introduced in Scotland in March 2006, there was a 17% reduction in hospital admissions for acute coronary syndrome. 67% of the decrease occurred in non-smokers.[21]
Treatment
People with presumed ACS are typically treated with aspirin, clopidogrel or ticagrelor, nitroglycerin, and if the chest discomfort persists morphine.[22] Other analgesics such as nitrous oxide are of unknown benefit.[22] Angiography is recommended in those who have either new ST elevation or a new left or right bundle branch block on their ECG.[1] Unless the person has low oxygen levels additional oxygen does not appear to be useful.[23]
STEMI
If the ECG confirms changes suggestive of myocardial infarction (ST elevations in specific leads, a new left bundle branch block or a true posterior MI pattern), thrombolytics may be administered or primary coronary angioplasty may be performed. In the former, medication is injected that stimulates fibrinolysis, destroying blood clots obstructing the coronary arteries. In the latter, a flexible catheter is passed via the femoral or radial arteries and advanced to the heart to identify blockages in the coronaries. When occlusions are found, they can be intervened upon mechanically with angioplasty and usually stent deployment if a lesion, termed the culprit lesion, is thought to be causing myocardial damage. Data suggest that rapid triage, transfer and treatment is essential.[24] The time frame for door-to-needle thrombolytic administration according to American College of Cardiology (ACC) guidelines should be within 30 minutes, whereas the door-to-balloon Percutaneous Coronary Intervention (PCI) time should be less than 90 minutes. It was found that thrombolysis is more likely to be delivered within the established ACC guidelines among patients with STEMI as compared to PCI according to a case control study.[25]
NSTEMI and NSTE-ACS
If the ECG does not show typical changes, the term "non-ST segment elevation ACS" is applied. The patient may still have had a "non-ST elevation MI" (NSTEMI). The accepted management of unstable angina and acute coronary syndrome is therefore empirical treatment with aspirin, a second platelet inhibitor such as clopidogrel, prasugrel or ticagrelor, and heparin (usually a low-molecular weight heparin), with intravenous nitroglycerin and opioids if the pain persists. The heparin-like drug known as fondaparinux appears to be better than enoxaparin.[26]
A blood test is generally performed for cardiac troponins twelve hours after onset of the pain. If this is positive, coronary angiography is typically performed on an urgent basis, as this is highly predictive of a heart attack in the near-future. If the troponin is negative, a treadmill exercise test or a thallium scintigram may be requested.
If there is no evidence of ST segment elevation on the electrocardiogram, delaying urgent angioplasty until the next morning is not inferior to doing so immediately.[27] Using statins in the first 14 days after ACS reduces the risk of further ACS.[28]
In a cohort study comparing NSTEMI and STEMI, people with NSTEMI had a similar risk of death at one year after PCI as compared to people with STEMI (3.4% vs 4.4%).[29] However, NSTEMI had significantly more "major cardiac events" (death, myocardial infarction, disabling stroke, or requiring revascularization) at one year (24.0% vs 16.6%).
Cocaine-associated ACS should be managed in a manner similar to other patients with acute coronary syndrome except beta blockers should not be used and benzodiazepines should be administered early.[30]
Prognosis
TIMI score
The TIMI risk score can identify high risk patients in non-ST segment elevation MI ACS[31] and has been independently validated.[32][33]
Global Registry of Acute Coronary Events (GRACE) score
Based on a global registry of 102,341 patients, the GRACE Risk Score estimates in-hospital, 6 months, 1 year, and 3-year mortality risk after a heart attack. GRACE Score 2.0 Calculator.[20]
Killip class
The Killip classification consists of 4 classes based on clinical symptoms. It predicts 30-day mortality after myocardial infarction.[34]
Biomarkers for diagnosis
The aim of diagnostic markers is to identify patients with ACS even when there is no evidence of heart muscle damage.
- Ischemia-Modified Albumin (IMA) – In cases of Ischemia – Albumin undergoes a conformational change and loses its ability to bind transitional metals (copper or cobalt). IMA can be used to assess the proportion of modified albumin in ischemia. Its use is limited to ruling out ischemia rather than a diagnostic test for the occurrence of ischemia.
- Myeloperoxidase (MPO) – The levels of circulating MPO, a leukocyte enzyme, elevate early after ACS and can be used as an early marker for the condition.
- Glycogen Phosphorylase Isoenzyme BB-(GPBB) is an early marker of cardiac ischemia and is one of three isoenzyme of Glycogen Phosphorylase.
- Troponin is a late cardiac marker of ACS
Biomarkers for risk determination
The aim of prognostic markers is to reflect different components of pathophysiology of ACS. For example:
- Natriuretic peptide – Both B-type natriuretic peptide (BNP) and N-terminal Pro BNP can be applied to predict the risk of death and heart failure following ACS.
- Monocyte chemo attractive protein (MCP)-1 – has been shown in a number of studies to identify patients with a higher risk of adverse outcomes after ACS.
Day of admission
Studies have shown that for ACS patients, weekend admission is associated with higher mortality and lower utilization of invasive cardiac procedures, and those who did undergo these interventions had higher rates of mortality and complications than their weekday counterparts. This data leads to the possible conclusion that access to diagnostic/interventional procedures may be contingent upon the day of admission, which may impact mortality.[35][36] This phenomenon is described as weekend effect.
Valvular heart disease
Valvular heart disease is characterized by damage to or defective in one of the four heart valves: the mitral, aortic, tricuspid or pulmonary.[37] Some types of valvular heart disease include valvular stenosis, valvular prolapse and regurgitation.
Oral manifestations
Oral infections may pose risk during postoperative period of heart valve surgery. Oral health in patients scheduled for heart valve surgery is poorer than in individuals without valve disease.[38] Most of them experience periodontitis due to high dental plaque scores, reflecting poorer dental hygiene. This situation could favour the appearance of bacteremia following tooth brushing in these individuals. Bacteremia secondary to periodontal infection is known to be one of the primary causes of infectious endocarditis, particularly in patient with heart valve disorders.[39] Therefore, treatment of dental disease should be done prior to performing heart surgery. Periodontal treatment is advised in patients with advanced periodontitis, followed by root planing and ultrasound treatment. Those teeth not amenable to treatment and with poor prognosis should be removed as pre-surgical preventive measures.
Dental management
The two main concerns during dental treatment for people of patient with valvular heart disease are the risk of infective endocarditis and bleeding in anti coagulated patients. Endocarditis is more likely to occur in patients who have previously had endocarditis and those with certain cardiac lesions. Risk of a normally functioning prosthesis being infected after a dental procedure is probably no higher than risk in patient with damaged native valves. However, mortality and morbidity is much higher should prosthesis become infected. Patient with native valve disease can often stop or reduce their anticoagulants, but those with prosthetic valves should not discontinue anticoagulants without cardiological advice. Mechanical mitral valves are prone to thrombosis, which cause emboli if adequate anti-coagulation is not maintained, although short term modification may be possible.[40]
Heart failure
Heart failure (HF) is defined as the incapacity of the heart to function properly, pumping insufficient blood towards the tissues and leading to fluid accumulation within the lungs, liver and peripheral tissues.
Oral manifestations
Most if not all patients with heart failure will be undergoing drug treatments for their condition and these drugs can produce a series of oral manifestation. In this context, angiotensin-converting enzyme (ACE) inhibitors such as captopril and enalapril can produce burning mouth sensation lichenoid reactions and a loss of taste sensation, while diuretics like furosemide can produce xerostomia.[41]
Dental management
Consultation with the supervising physician is highly advised in order to understand the patient's current condition and the medication prescribed. The patient should be receiving medical care, and heart failure should be compensated.[42] Dental treatment is to be limited to patients who are in stable condition, since these people are at a high risk of developing questionable arrhythmias and even sudden death secondary to cardiopulmonary arrest. Stress and anxiety are to be avoided during the visits, which in turn should be brief (< 30 minutes) and are to be scheduled for the morning sessions. The patient should be seated on the chair in a semi-supine position, with control of body movements (which should be slow), to avoid orthostatic hypotension. In patients who has been administered with digitalis agents (digoxin, methyl-digoxin), the vasoconstrictor dose should be limited to two anaesthetic carpules, since this drug combination can cause arrhythmias.[43] Aspirin (acetylsalicylic acid) can lead to fluid and sodium retention, and therefore should not be prescribed in patients with heart failure.
In emergency (i.e., lung edema), after contacting the emergency service, the patient should be seated with the legs lowered, and receiving nasal oxygen at a rate of 4–6 liters/minute. Sublingual nitroglycerin tablets are indicated (0.4-0.8 mg), and the dose may be repeated every 5 or 10 minutes if blood pressure is maintained.
Arrhythmia
Arrhythmias are variations in normal heart rate due to cardiac rhythm, frequency or contraction disorders. The most common type of cardiac arrhythmia is atrial fibrillation.
Oral manifestations
Many anti-arrhythmic drugs have side effects such as gingival hyperplasia or xerostomia.
Dental management
Consultation with the supervising physician is also advised in order to understand the patient's current condition and the type of arrhythmia involved, as well as the medication prescribed. It must be checked that the patient uses the medication correctly. Stress and anxiety can be reduced with anxiolytics. Short visits in the morning are to be preferred.[44] Patient monitoring, with recording of the pulse, is indicated before treatment. It is very important to limit the use of vasoconstrictor in local anesthesia, with no more than two carpules. The treatment planned should not be too long or complicated. Although modern pacemakers are more resistant to electromagnetic interferences, caution is required when using electrical devices like ultrasound and electric scalpels that might interfere with pacemakers – especially the older models, since such devices developed in the last 30 years are bipolar and are generally not affected by the small electromagnetic fields generated by dental equipment. It is therefore important to know the type of pacemaker, the degree of electromagnetic protection of the generator, and the nature of the arrhythmia. If arrhythmia develops during dental treatment, the procedure should be suspended, oxygen is to be given, and the patient vital signs are to be assessed: body temperature (normal values: 35.5-37oC), pulse (normal values: 60-100 bpm), respiratory frequency (normal values in adults: 14-20 cycles or respirations per minute), blood pressure (normal values: systolic blood pressure under 140 mmHg and diastolic blood pressure under 90 mmHg). Sublingual nitrites are to be administered if there is chest pain.[45] The patient should be placed in the Trendelenburg position, with vagal maneuvering where necessary (valsalva maneuver, massage in the carotid pulse region).[46] The dental team should be prepared for basic cardiopulmonary resuscitation and initiation of the emergency procedure for evacuation to a hospital centre, if necessary.
See also
- Allergic acute coronary syndrome (Kounis syndrome)
References
- 1 2 Amsterdam, E. A.; Wenger, N. K.; Brindis, R. G.; Casey, D. E.; Ganiats, T. G.; Holmes, D. R.; Jaffe, A. S.; Jneid, H.; Kelly, R. F.; Kontos, M. C.; Levine, G. N.; Liebson, P. R.; Mukherjee, D.; Peterson, E. D.; Sabatine, M. S.; Smalling, R. W.; Zieman, S. J. (23 September 2014). "2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines". Circulation. 130 (25): e344–e426. doi:10.1161/CIR.0000000000000134. PMID 25249585.
- ↑ Goodacre S, Pett P, Arnold J, Chawla A, Hollingsworth J, Roe D, Crowder S, Mann C, Pitcher D, Brett C (November 2009). "Clinical diagnosis of acute coronary syndrome in patients with chest pain and a normal or non-diagnostic electrocardiogram". Emergency Medicine Journal. 26 (12): 866–870. doi:10.1136/emj.2008.064428. PMID 19934131. Archived from the original on 5 April 2017.
- ↑ Canto JG, Shlipak MG, Rogers WJ (June 2000). "Prevalence, Clinical Characteristics, and Mortality among Patients with Acute Myocardial Infarction Presenting Without Chest Pain". JAMA. 283 (24): 3223–3229, vi. doi:10.1001/jama.283.24.3223. PMID 10866870.
- ↑ Grech ED, Ramsdale DR (June 2003). "Acute coronary syndrome: unstable angina and non-ST segment elevation myocardial infarction". BMJ. 326 (7401): 1259–61. doi:10.1136/bmj.326.7401.1259. PMC 1126130. PMID 12791748.
- ↑ Torres M, Moayedi S (May 2007). "Evaluation of the acutely dyspneic elderly patient". Clin. Geriatr. Med. 23 (2): 307–25, vi. doi:10.1016/j.cger.2007.01.007. PMID 17462519.
- ↑ "Dorlands Medical Dictionary:acute coronary syndrome". Archived from the original on 7 September 2009.
- ↑ Woo KM, Schneider JI (November 2009). "High-risk chief complaints I: chest pain--the big three". Emerg. Med. Clin. North Am. 27 (4): 685–712, x. doi:10.1016/j.emc.2009.07.007. PMID 19932401.
- ↑ Achar SA, Kundu S, Norcross WA (2005). "Diagnosis of acute coronary syndrome". Am Fam Physician. 72 (1): 119–26. PMID 16035692. Archived from the original on 9 October 2007.
- ↑ "Chest Pain in the Emergency Department: Differential Diagnosis". The Cardiology Advisor. 20 January 2019. Archived from the original on 25 July 2019. Retrieved 25 July 2019.
- ↑ Kubo, Takashi; Ino, Yasushi; Tanimoto, Takashi; Kitabata, Hironori; Tanaka, Atsushi; Akasaka, Takashi (2011). "Optical Coherence Tomography Imaging in Acute Coronary Syndromes". Cardiology Research and Practice. 2011: 312978. doi:10.4061/2011/312978. ISSN 2090-8016. Retrieved 10 November 2022.
- 1 2 3 Eisen, Alon; Giugliano, Robert P; Braunwald, Eugene (20 July 2016). "Updates on acute coronary syndrome: A review". JAMA Cardiology. 1 (16): 718–730. doi:10.1001/jamacardio.2016.2049. PMID 27438381.
- ↑ Franke, Kyle B; Wong, Dennis TL; Baumann, Angus; Nicholls, Stephen J; Gulati, Rajiv; Psaltis, Peter J (4 April 2019). "Current state-of-play in spontaneous coronary artery dissection". Cardiovascular Diagnosis and Therapy. 9 (3): 281–298. doi:10.21037/cdt.2019.04.03. PMC 6603494. PMID 31275818. Archived from the original on 28 November 2021. Retrieved 3 November 2022.
- ↑ Tamis-Holland, JE (27 March 2019). "Diagnosis and Management of MINOCA Patients". Circulation. 139 (18): 891–908. doi:10.1161/CIR.0000000000000670. PMID 30913893.
- ↑ Alpert JS, Thygesen K, Antman E, Bassand JP (2000). "Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction". J Am Coll Cardiol. 36 (3): 959–69. doi:10.1016/S0735-1097(00)00804-4. PMID 10987628.
- ↑ Chun AA, McGee SR (2004). "Bedside diagnosis of coronary artery disease: a systematic review". Am. J. Med. 117 (5): 334–43. doi:10.1016/j.amjmed.2004.03.021. PMID 15336583.
- ↑ Neumar, RW; Shuster, M; Callaway, CW; Gent, LM; Atkins, DL; Bhanji, F; Brooks, SC; de Caen, AR; Donnino, MW; Ferrer, JM; Kleinman, ME; Kronick, SL; Lavonas, EJ; Link, MS; Mancini, ME; Morrison, LJ; O'Connor, RE; Samson, RA; Schexnayder, SM; Singletary, EM; Sinz, EH; Travers, AH; Wyckoff, MH; Hazinski, MF (3 November 2015). "Part 1: Executive Summary: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care". Circulation. 132 (18 Suppl 2): S315–67. doi:10.1161/cir.0000000000000252. PMID 26472989.
- ↑ Selker HP, Griffith JL, D'Agostino RB (1991). "A tool for judging coronary care unit admission appropriateness, valid for both real-time and retrospective use. A time-insensitive predictive instrument (TIPI) for acute cardiac ischemia: a multicenter study". Medical Care. 29 (7): 610–27. doi:10.1097/00005650-199107000-00002. PMID 2072767. S2CID 31892999.
- ↑ Goodacre, S; Pett, P; Arnold, J; Chawla, A; Hollingsworth, J; Roe, D; Crowder, S; Mann, C; Pitcher, D; Brett, C (2009). "Clinical diagnosis of acute coronary syndrome in patients with chest pain and a normal or non-diagnostic electrocardiogram". Emergency Medicine Journal. 26 (12): 866–70. doi:10.1136/emj.2008.064428. PMID 19934131.
- ↑ Sekhri, N; Feder, GS; Junghans, C; Eldridge, S; Umaipalan, A; Madhu, R; Hemingway, H; Timmis, AD (2008). "Incremental prognostic value of the exercise electrocardiogram in the initial assessment of patients with suspected angina: cohort study". BMJ (Clinical Research Ed.). 337: a2240. doi:10.1136/bmj.a2240. PMC 2583389. PMID 19008264.
- 1 2 Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, Avezum A, Goodman SG, Flather MD, Anderson FA Jr, Granger CB (2006). "Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE)". BMJ. 333 (7578): 1091. doi:10.1136/bmj.38985.646481.55. PMC 1661748. PMID 17032691.
- ↑ Pell JP, Haw S, Cobbe S, et al. (2008). "Smoke-free Legislation and Hospitalizations for Acute Coronary Syndrome" (PDF). New England Journal of Medicine. 359 (5): 482–91. doi:10.1056/NEJMsa0706740. hdl:1893/16659. PMID 18669427. Archived (PDF) from the original on 22 March 2020. Retrieved 3 November 2022.
- 1 2 O'Connor RE, Brady W, Brooks SC, et al. (November 2010). "Part 10: acute coronary syndromes: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care". Circulation. 122 (18 Suppl 3): S787–817. doi:10.1161/CIRCULATIONAHA.110.971028. PMID 20956226.
- ↑ Neumar, RW; Shuster, M; Callaway, CW; Gent, LM; Atkins, DL; Bhanji, F; Brooks, SC; de Caen, AR; Donnino, MW; Ferrer, JM; Kleinman, ME; Kronick, SL; Lavonas, EJ; Link, MS; Mancini, ME; Morrison, LJ; O'Connor, RE; Samson, RA; Schexnayder, SM; Singletary, EM; Sinz, EH; Travers, AH; Wyckoff, MH; Hazinski, MF (3 November 2015). "Part 1: Executive Summary: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care". Circulation. 132 (18 Suppl 2): S315–67. doi:10.1161/cir.0000000000000252. PMID 26472989.
- ↑ Blankenship JC, Skelding KA (2008). "Rapid Triage, Transfer, and Treatment with Percutaneous Coronary Intervention for Patients with ST-Segment Elevation Myocardial Infarction". Acute Coronary Syndromes. 9 (2): 59–65. Archived from the original on 15 July 2011.
- ↑ Janda, SP; Tan, N (2009). "Thrombolysis versus primary percutaneous coronary intervention for ST elevation myocardial infarctions at Chilliwack General Hospital". The Canadian Journal of Cardiology. 25 (11): e382–4. doi:10.1016/S0828-282X(09)70165-5. PMC 2776568. PMID 19898701.
- ↑ Bundhun, PK; Shaik, M; Yuan, J (8 May 2017). "Choosing between Enoxaparin and Fondaparinux for the management of patients with acute coronary syndrome: A systematic review and meta-analysis". BMC Cardiovascular Disorders. 17 (1): 116. doi:10.1186/s12872-017-0552-z. PMC 5422952. PMID 28482804.
- ↑ Montalescot G, Cayla G, Collet JP, Elhadad S, Beygui F, Le Breton H, et al. (2009). "Immediate vs delayed intervention for acute coronary syndromes: a randomized clinical trial". JAMA. 302 (9): 947–54. doi:10.1001/jama.2009.1267. PMID 19724041.
- ↑ Vale, N; Nordmann, AJ; Schwartz, GG; de Lemos, J; Colivicchi, F; den Hartog, F; Ostadal, P; Macin, SM; Liem, AH; Mills, EJ; Bhatnagar, N; Bucher, HC; Briel, M (1 September 2014). "Statins for acute coronary syndrome". The Cochrane Database of Systematic Reviews. 9 (9): CD006870. doi:10.1002/14651858.CD006870.pub3. PMID 25178118.
- ↑ Cox, D. A.; Stone, G. W.; Grines, C. L.; Stuckey, T.; Zimetbaum, P. J.; Tcheng, J. E.; Turco, M.; Garcia, E.; Guagliumi, G.; Iwaoka, R. S.; Mehran, R.; O'Neill, W. W.; Lansky, A. J.; Griffin, J. J.; Cadillac, I. (2006). "Comparative Early and Late Outcomes After Primary Percutaneous Coronary Intervention in ST-Segment Elevation and Non–ST-Segment Elevation Acute Myocardial Infarction (from the CADILLAC Trial)". The American Journal of Cardiology. 98 (3): 331–337. doi:10.1016/j.amjcard.2006.01.102. PMID 16860018.
- ↑ McCord J, Jneid H, Hollander JE, et al. (April 2008). "Management of cocaine-associated chest pain and myocardial infarction: a scientific statement from the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology". Circulation. 117 (14): 1897–907. doi:10.1161/CIRCULATIONAHA.107.188950. PMID 18347214.
- ↑ Antman EM, Cohen M, Bernink PJ, et al. (2000). "The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making". JAMA. 284 (7): 835–42. doi:10.1001/jama.284.7.835. PMID 10938172.
- ↑ Pollack CV, Sites FD, Shofer FS, Sease KL, Hollander JE (2006). "Application of the TIMI risk score for unstable angina and non-ST elevation acute coronary syndrome to an unselected emergency department chest pain population". Academic Emergency Medicine. 13 (1): 13–8. doi:10.1197/j.aem.2005.06.031. PMID 16365321.
- ↑ Chase M, Robey JL, Zogby KE, Sease KL, Shofer FS, Hollander JE (2006). "Prospective validation of the Thrombolysis in Myocardial Infarction Risk Score in the emergency department chest pain population". Annals of Emergency Medicine. 48 (3): 252–9. doi:10.1016/j.annemergmed.2006.01.032. PMID 16934646.
- ↑ Killip, Thomas; Kimball, John T. (1967). "Treatment of myocardial infarction in a coronary care unit". The American Journal of Cardiology. 20 (4): 457–464. doi:10.1016/0002-9149(67)90023-9. PMID 6059183.
- ↑ Khoshchehreh M, Groves EM, Tehrani D, Amin A, Patel PM, Malik S (2016). "Changes in mortality on weekend versus weekday admissions for Acute Coronary Syndrome in the United States over the past decade". Int J Cardiol. 210: 164–172. doi:10.1016/j.ijcard.2016.02.087. PMC 4801736. PMID 26950171.
- ↑ Kostis W.J.; Demissie K.; Marcella S.W.; Shao Y.-H.; Wilson A.C.; Moreyra A.E. (2007). "Weekend versus weekday admission and mortality from myocardial infarction". N Engl J Med. 356 (11): 1099–1109. doi:10.1056/nejmoa063355. PMID 17360988.
- ↑ [Valvular heart disease is characterized by damage to or defective in one of the four heart valves: the mitral, aortic, tricuspid or pulmonary. "Valvular Heart Disease"]. "Valvular Heart Disease".
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: multiple names: authors list (link) - ↑ Silvestre, FJ; Gil-Raga, I; Martinez-Herrera, M; Lauritano, D; Silvestre-Rangil, J (2017). "Prior oral conditions in patients undergoing heart valve surgery". Journal of Clinical and Experimental Dentistry. 9 (11): e1287–e1291. doi:10.4317/jced.53902. ISSN 1989-5488. PMC 5741840. PMID 29302279.
- ↑ Jowett, N.; Cabot, L. (23 September 2000). "Patients with cardiac disease: considerations for the dental practitioner". British Dental Journal. 189 (6): 297–302. doi:10.1038/sj.bdj.4800750a. ISSN 0007-0610. PMID 11060950.
- ↑ ROSE, LOUIS F.; MEALEY, BRIAN; MINSK, LAURA; COHEN, D. WALTER (2002). "Oral care for patients with cardiovascular disease and stroke". The Journal of the American Dental Association. 133: 37S–44S. doi:10.14219/jada.archive.2002.0378. ISSN 0002-8177. PMID 12085723.
- ↑ Cáceres, Maria Teresa Fernández; Ludovice, Ana Cristina P. P.; Brito, Fabio Sândoli de; Darrieux, Francisco Carlos; Neves, Ricardo Simões; Scanavacca, Mauricio Ibrahim; Sosa, Eduardo A.; Hachul, Denise Tessariol (2008). "Efeito de anestésicos locais com e sem vasoconstritor em pacientes com arritmias ventriculares". Arquivos Brasileiros de Cardiologia. 91 (3): 128–33, 142–7. doi:10.1590/s0066-782x2008001500002. ISSN 0066-782X. PMID 18853053.
- ↑ MUZYKA, BRIAN C. (1999). "Atrial Fibrillation and ITS Relationship to Dental Care". The Journal of the American Dental Association. 130 (7): 1080–1085. doi:10.14219/jada.archive.1999.0339. ISSN 0002-8177. PMID 10422402.
- ↑ Magarakis, Michael; Macias, Alejandro E.; Ghodsizad, Ali; Salerno, Tomas A. (2018), "Surgical Management of Cardiovascular Thrombotic Conditions", Cardiovascular Thrombus, Elsevier, pp. 367–376, doi:10.1016/b978-0-12-812615-8.00025-9, ISBN 978-0-12-812615-8
- ↑ Gourraud, Jean-Baptiste; Barc, Julien; Thollet, Aurélie; Le Marec, Hervé; Probst, Vincent (2017). "Brugada syndrome: Diagnosis, risk stratification and management". Archives of Cardiovascular Diseases. 110 (3): 188–195. doi:10.1016/j.acvd.2016.09.009. ISSN 1875-2136. PMID 28139454.
- ↑ McDonald, Jay R. (2009). "Acute Infective Endocarditis". Infectious Disease Clinics of North America. 23 (3): 643–664. doi:10.1016/j.idc.2009.04.013. ISSN 0891-5520. PMC 2726828. PMID 19665088.
- ↑ Chaudhry, Swantika; Jaiswal, Ritika; Sachdeva, Surender (2016). "Dental considerations in cardiovascular patients: A practical perspective". Indian Heart Journal. 68 (4): 572–575. doi:10.1016/j.ihj.2015.11.034. ISSN 0019-4832. PMC 4990738. PMID 27543484.
External links
| Classification | |
|---|---|
| External resources |