Libman–Sacks endocarditis
| Libman–Sacks endocarditis | |
|---|---|
| Other names: Nonbacterial thrombotic endocarditis, Marantic endocarditis, verrucous endocarditis | |
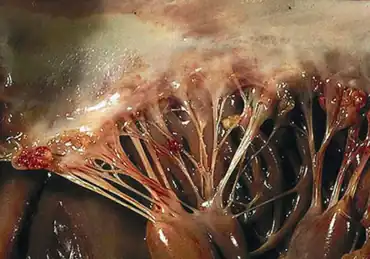 | |
| Verrucous vegetations of the mitral valve-Libman–Sacks endocarditis | |
| Specialty | Rheumatology, Cardiology |
| Causes | Systemic lupus erythematosus, Malignancy, Antiphospholipid syndrome |
| Diagnostic method | Echocardiography |
Libman–Sacks endocarditis (LSE) is a form of non-infective endocarditis that is seen in association with systemic lupus erythematosus (SLE), antiphospholipid syndrome, and cancers.[1]
Signs and symptoms
LSE itself is typically asymptomatic. Affected persons most commonly present with embolisms secondary to dislodged vegetations. However, in some cases, severe valvular dysfunction may develop. People with SLE may present with other symptoms of the underlying diseases that give rise to LSE. [2]
Complications
More severe LSE may result in arterial emboli, valvular insufficiency, and heart failure. Infective endocarditis occurs more frequently with those with SLE.[3]
Emboli
Vegetations occurring in the context of LSE may dislodge to form emboli and cause embolism (including cerebral embolism (presenting as stroke or transient ischaemic attack), mesenteric ischaemia (presenting with severe abdominal pain), or peripheral arterial embolism (presenting with limb coldness)).[1]
Causes
LSE occurs in association with systemic lupus erythematosus, antiphospholipid syndrome, and malignancies.[1]
Systemic lupus erythematosus
In SLE, LSE has been linked to pericarditis, presence of anticardiolipin antibodies, arterial and venous thromboses, and neuropsychiatric manifestations of SLE. SLE is associated with greater SLE duration and severity. In some cases, LSE may be the presenting pathology in SLE, especially in the presence of concurrent antiphospholipid syndrome.[3]
Pathophysiology
The initial cause of LSE is poorly understood. LSE is thought to occur in the context of a hypercoagulable state which leads to endothelial injury and subsequent deposition of thrombi and inflammatory molecules in affected valves. The vegetations that are thus formed consist of immune complexes, platelet thrombi, fibrin, and mononuclear cells. The vegetations may dislodge and cause embolisms.[1]
Histopathology
LSE involves formation of cardiac lesions that may take the form of vegetations or thickening of the valvular leaflets.[1]
The vegetations are small and formed from strands of fibrin, neutrophils, lymphocytes, and histiocytes.[4] Vegetations are most often small-to-moderate in size (<10mm),[3] but may sometimes be large (>10mm).[1] The mitral valve is typically affected, and the vegetations occur on the ventricular and atrial surface of the valve.[4] Though the left-sided heart valves (mitral and aortic) are most commonly affected, any of the heart valves as well as adjoining structures may become involved.[3]
Libman–Sacks lesions rarely produce significant valve dysfunction and the lesions only rarely embolize.[4] However, there is data to suggest an association between Libman–Sacks endocarditis and a higher risk for embolic cerebrovascular disease in people with SLE.[2]
Diagnosis
LSE should be considered in instances of thromboembolic event in persons with underlying pathology that is associated with LSE. LSE is diagnosed with echocardiography. Other potential etiologies (e.g. infective endocarditis) should be excluded through an extensive assessment (complete blood count and metabolic panel, blood cultures). LSE can also be identified post-mortem during an autopsy.[1]
Echocardiography
Echocardiography is considered the primary evaluation for LSE; transesophageal echocardiography (TEE) has greater sensitivity and specificity than transthoracic echocardiography (TTE).[1][3] In case of a negative TTE in the presence of clinical signs of LSE, TEE may be attempted to confirm the presence of the condition.[3]
Vegetations of the cardiac valves and endocardium are characterised by irregular borders, heterogenous echo density, and an absence of independent motion. Vegetations are usually small, but may be as large as 10mm. The basal and middle portions of the mitral and aortic valves are most commonly involved. Leaflet thickening or regurgitation may be present. There may be other cardiac pathology related to the underlying cause (SLE).[1]
Differential diagnosis
Differential diagnoses include: rheumatic valvular disease, atrial myxoma, degenerative valvular disease, infective endocarditis, vasculitis, cholesterol emboli syndrome, fibroelastoma, and Lambl's excrescences.[1]
Management/treament
The condition should be monitored to follow the development of the vegetations, and health personnel should be conscious of the potential risks associated with the condition.[1]
There is a paucity of empirical evidence on treatment options for persons with LSE, and treatment should focus on the underlying cause. Anticoagulant treatment is recommended in cases with previous thromboembolic event for prevention of subsequent occurrences. Surgical intervention may be indicated in case of significant valvular dysfunction.[1]
Prognosis
LSE is often associated with considerable morbidity and mortality.[1]
Epidemiology
LSE has been observed in 0.2% in of the general population at autopsy. It occurs most commonly in those aged 40-80 years.[1]
LSE vegetations are observed in 10% of SLE cases (however, in one study, vegetations were noted in 43% of SLE cases (0% in controls), and valvular thickening in 51% of SLE cases (7% in controls)[3]).[1] There is a significant correlation between SLE duration and severity, and the incidence of LSE.[1][3]LSE has been identified in 1.25% of those with malignant disease at autopsy.[1]
History
It was first described by Emanuel Libman and Benjamin Sacks at Mount Sinai Hospital in New York City in 1924.[5][6] The association between Libman–Sacks endocarditis and antiphospholipid syndrome was first noted in 1985.[7]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Ibrahim, Abdisamad M.; Siddique, Momin S. (2020), "Libman Sacks Endocarditis", StatPearls, StatPearls Publishing, PMID 30422459, archived from the original on 2021-09-22, retrieved 2020-05-30
- 1 2 Roldan, C. A.; Sibbitt, W. L. Jr (2013). "Libman–Sacks endocarditis and embolic cerebrovascular disease". Cardiovascular Imaging. 6 (9): 973–983. doi:10.1016/j.jcmg.2013.04.012. PMC 3941465. PMID 24029368.
- 1 2 3 4 5 6 7 8 Dubois' lupus erythematosus and related syndromes. Wallace, Daniel J. (Daniel Jeffrey), 1949-, Hahn, Bevra (Ninth ed.). Edinburgh. 16 August 2018. ISBN 978-0-323-55064-2. OCLC 1051140253.
{{cite book}}: CS1 maint: others (link) - 1 2 3 Doherty, N. E.; Siegel, R. J. (1985). "Cardiovascular manifestations of systemic lupus erythematosus". Am Heart J. 110 (6): 1257–1265. doi:10.1016/0002-8703(85)90023-7. PMID 3907317.
- ↑ Libman, E.; Sacks, B. (1924). "A hitherto undescribed form of valvular and mural endocarditis". Arch Intern Med. 33 (6): 701–737. doi:10.1001/archinte.1924.00110300044002.
- ↑ "Patient.info: Libman–Sacks Endocarditis". Archived from the original on 2015-08-01. Retrieved 2008-08-11.
- ↑ Hojnik Maja; George Jacob; Ziporen Lea; Shoenfeld Yehuda (1996-04-15). "Heart Valve Involvement (Libman-Sacks Endocarditis) in the Antiphospholipid Syndrome". Circulation. 93 (8): 1579–1587. doi:10.1161/01.CIR.93.8.1579. PMID 8608627.
External links
| Classification | |
|---|---|
| External resources |