Mipomersen
 | |
| Names | |
|---|---|
| Trade names | Kynamro |
| Other names | ISIS 301012 |
IUPAC name
| |
| Clinical data | |
| Drug class | Antisense oligonucleotide[1] |
| Main uses | Homozygous familial hypercholesterolemia[2] |
| Side effects | Tiredness, nausea, headache, liver problems, fever, high blood pressure, abdominal pain[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Subcutaneous injection |
| Typical dose | 200 mg q wk[2] |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Protein binding | ≥90% |
| Metabolism | Nucleases |
| Elimination half-life | 1–2 months |
| Excretion | <4% in urine in 24 hours |
| Chemical and physical data | |
| Formula | C230H305N67Na19O122P19S19 |
| Molar mass | 7594.76 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Mipomersen, sold under the brand name Kynamro, is a medication used to treat homozygous familial hypercholesterolemia.[2] Effects on overall risk of heart disease or death is unclear.[2] Benefits in other types of high cholesterol is unknown.[2] It is given by injection under the skin.[2]
Common side effects include tiredness, nausea, headache, liver problems, fever, high blood pressure, and abdominal pain.[2] Other side effects may include pain at the site of injection and angioedema.[2] It is a short fragment of DNA, known as an antisense oligonucleotide, that blocks the production of apolipoprotein B.[1]
Mipomersen was approved for medical use in the United States in 2013.[2] In Europe it was denied approval in 2013 due to concerns of greater side effects than benefits.[1] In the United States it costs about 407,000 USD per year.[3]
Medical uses
Kynamro is used to treat homozygous familial hypercholesterolemia and is administered by injection.[4][5]
Dosage
The typical dose is 200 mg per week.[2]
In the United States one must enrol in a Risk Evaluation and Mitigation Strategies (REMS) program approved by the FDA.[4]
Contraindications
The drug is contraindicated in people with moderate to severe liver impairment, active liver diseases, and unexplained high levels of transaminase liver enzymes.[4][6]
Side effects
The drug has a black box warning about the risk of liver damage; specifically it can cause elevations in the levels of transaminases and causes fatty liver disease.[4]
In clinical trials, 18% of subjects taking mipomersen stopped using the drug due to adverse effects; the most common adverse effects leading to discontinuation were injection site reactions, increases of transaminases, flu-like symptoms (fever, chills, abdominal pain, nausea, vomiting), and abnormal liver tests.[4]
Other adverse effects include: heart problems including angina and palpitations, edema, pain in legs or arms, headache, insomnia, and hypertension.[4]
Pregnancy and breastfeeding
Mipomersen is pregnancy category B; women who are pregnant or intending to become pregnant should only use this drug if needed. It is unknown if it is secreted in human breast milk, but it was found to be secreted in the breast milk of rats.[4]
Interactions
Other drugs known for causing liver problems might add to mipomersen's risk of liver damage. No pharmacokinetic interactions have been described.[6]
Pharmacology
Mechanism of action
Mipomersen binds to the messenger RNA coding for apolipoprotein B-100 (ApoB-100), a protein that is the main component of low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL). As a consequence, the RNA is degraded by the enzyme ribonuclease H, and ApoB-100 is not translated.[6]
Pharmacokinetics
After subcutaneous injection, mipomersen reaches highest blood levels after 3 to 4 hours. It accumulates in the liver, which is convenient since apolipoprotein B predominantly acts there. Protein binding is over 90%. The molecule is slowly broken up by endonucleases and subsequently by exonucleases. After 24 hours, less than 4% of the degradation products are found in the urine, and overall half-life is 1 to 2 months.[6]
Chemistry

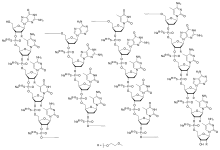
The compound is a 'second-generation' antisense oligonucleotide; the nucleotides are linked with phosphorothioate linkages rather than the phosphodiester linkages of RNA and DNA, and the sugar parts are deoxyribose in the middle part of the molecule and 2’-O-methoxyethyl-modified ribose at the two ends. These modifications make the drug resistant to degradation by nucleases, allowing it to be administered weekly.
The complete sequence is portrayed below:[8][4]: 10
5’—G*—mC*—mC*—mU*—mC*—dA—dG—dT—dmC—dT—dG—dmC—dT—dT—dmC—G*—mC*—A*—mC*—mC*—3’*= 2’-O-(2-methoxyethyl)m= 5-methyld= 2’-deoxy
History
The drug was discovered and developed to Phase 2 by Ionis Pharmaceuticals and subsequently licensed to Genzyme Corporation in 2008 by an auction bid. Ionis earned an upfront payment of $325 million, with payments of a further $825 million if milestones are met.[9]
Mipomersen was rejected by the European Medicines Agency in 2012[10] and again in 2013 due to concerns about the liver and cardiovascular adverse effects.[11]
In January 2013, The United States Food and Drug Administration approved mipomersen for the treatment of homozygous familial hypercholesterolemia.[12][13]
References
- 1 2 3 "Kynamro". Archived from the original on 8 March 2021. Retrieved 18 November 2021.
- 1 2 3 4 5 6 7 8 9 10 11 "Mipomersen Monograph for Professionals". Drugs.com. Archived from the original on 24 January 2021. Retrieved 18 November 2021.
- ↑ "Kynamro Prices, Coupons & Patient Assistance Programs". Drugs.com. Retrieved 18 November 2021.
- 1 2 3 4 5 6 7 8 9 "Kynamro (mipomersen sodium) Injection, Solution for Subcutaneous Injection. Full Prescribing Information" (PDF). Kastle Therapeutics, Chicago, IL. Archived (PDF) from the original on 2020-02-06. Retrieved 2021-09-17.
- ↑ Feingold, KR; Grunfeld, C (August 10, 2016). "Cholesterol Lowering Drugs". In De Groot, LJ; et al. (eds.). Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc. PMID 27809434. Archived from the original on October 31, 2021. Retrieved September 17, 2021.
- 1 2 3 4 Drugs.com: Mipomersen Professional Drug Facts.
- ↑ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 61" (PDF). World Health Organization. 2009. Archived (PDF) from the original on 2020-10-03. Retrieved 2021-09-17.
- ↑ Geary RS, Baker BF, Crooke ST (February 2015). "Clinical and preclinical pharmacokinetics and pharmacodynamics of mipomersen (kynamro(®)): a second-generation antisense oligonucleotide inhibitor of apolipoprotein B". Clinical Pharmacokinetics. 54 (2): 133–146. doi:10.1007/s40262-014-0224-4. PMC 4305106. PMID 25559341.
- ↑ "Genzyme and Isis Complete Licensing of Mipomersen". 24 June 2008. Archived from the original on 29 September 2011.
- ↑ "EMA committee shoots down Sanofi's cholesterol drug mipomersen". FierceBiotech. December 14, 2012. Archived from the original on June 28, 2021. Retrieved September 17, 2021.
- ↑ "Refusal of the marketing authorisation for Kynamro (mipomersen)" (PDF). EMA. March 21, 2013. Archived (PDF) from the original on June 15, 2018. Retrieved September 17, 2021.
- ↑ Pollack, Andrew (29 January 2013) F.D.A. Approves Genetic Drug to Treat Rare Disease Archived 2018-06-24 at the Wayback Machine The New York Times, Retrieved 31 January 2013
- ↑ Staff (29 January 2013) FDA approves new orphan drug Kynamro to treat inherited cholesterol disorder Archived 2013-02-02 at the Wayback Machine U.S. Food and Drug Administration, Retrieved 31 January 2013
External links
| Identifiers: |
|---|