Degarelix
 | |
| Names | |
|---|---|
| Trade names | Firmagon, others |
| Other names | FE-200486 |
| Clinical data | |
| Drug class | GnRH analogue; GnRH antagonist; Antigonadotropin |
| Main uses | Prostate cancer[1] |
| Side effects | Pain at site of injection, hot flushes, liver problems[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category | |
| Routes of use | Subcutaneous injection |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609022 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | 30–40% |
| Protein binding | ~90% |
| Metabolism | Subject to common peptidic degradation during passage through the hepato-biliary system; not a substrate for the human CYP450 system |
| Elimination half-life | 23–61 days |
| Excretion | Feces: 70–80% Urine: 20–30% |
| Chemical and physical data | |
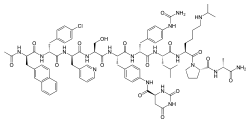
| Formula | C82H103ClN18O16 |
| Molar mass | 1632.29 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Degarelix, sold under the brand name Firmagon among others, is a medication used to treat prostate cancer.[1][4] Specifically it is used for advanced disease that is hormone dependent.[5] It is given by injection under the skin.[5]
Common side effects include pain at the site of injection, hot flushes, and liver problems.[1] Other side effects may include anaphylaxis, QT prolongation, and infertility.[1] Use in pregnancy may harm the baby.[1] It is a GnRH receptor antagonist and works by reducing the amount of testosterone in the body.[1][6]
Degarelix was approved for medical use in the United States in 2008 and Europe in 2009.[4][6] In the United Kingdom 80 mg cost the NHS about £130 as of 2021.[5] In the United States this amount costs about 520 USD.[7]
Medical uses
Degarelix, through its ability to reduce serum testosterone, is used to treat hormone-sensitive prostate cancer.[8]
Dosage
It is given at an initial dose of 240 mg followed by 80 mg every 4 weeks.[5]
Side effects
As with all hormonal therapies, degarelix is commonly associated with hormonal side effects such as hot flashes and weight gain.[8][9][10] Due to its mode of administration (subcutaneous injection), degarelix is also associated with injection-site reactions such as injection-site pain, erythema or swelling. Injection-site reactions are usually mild or moderate in intensity and occur predominantly after the first dose, decreasing in frequency thereafter.[8] Less common: Anemia. Diarrhea, nausea. Hyperhidrosis including night sweats, rash. Gynecomastia, testicular atrophy, erectile dysfunction. Increased transaminases. Musculoskeletal pain and discomfort. Dizziness, headache. Insomnia. Weight gain. Chills, fever, fatigue, flu-like illness.[11]
Pharmacology
GnRH antagonists (receptor blockers) such as degarelix are synthetic peptide derivatives of the natural GnRH decapeptide – a hormone that is made by neurons in the hypothalamus. GnRH antagonists compete with natural GnRH for binding to GnRH receptors in the pituitary gland. This reversible binding blocks the release of LH and FSH from the pituitary. The reduction in LH subsequently leads to a rapid and sustained suppression of testosterone release from the testes and subsequently reduces the size and growth of the prostate cancer. This in turn results in a reduction in prostate-specific antigen (PSA) levels in the patient's blood. Measuring PSA levels helps to monitor how patients with prostate cancer are responding to treatment.
Unlike GnRH agonists, which cause an initial stimulation of the hypothalamic-pituitary-gonadal axis (HPGA), leading to a surge in testosterone levels, and under certain circumstances, a flare-up of the tumour, GnRH antagonists do not cause a surge in testosterone or clinical flare.[12] Clinical flare is a phenomenon that occurs in patients with advanced disease, which can precipitate a range of clinical symptoms such as bone pain, urethral obstruction, and spinal cord compression. Drug agencies have issued boxed warnings regarding this phenomenon in the prescribing information for GnRH agonists. As testosterone surge does not occur with GnRH antagonists, there is no need for patients to receive an antiandrogen as flare protection during prostate cancer treatment. GnRH agonists also induce an increase in testosterone levels after each reinjection of the drug – a phenomenon that does not occur with GnRH antagonists such as degarelix.
GnRH antagonists have an immediate onset of action leading to a fast and profound suppression of testosterone and are therefore especially valuable in the treatment of patients with prostate cancer where fast control of disease is needed.
History
In December 2008, the US Food and Drug Administration (FDA) approved degarelix for the treatment of patients with advanced prostate cancer in the United States.[13][14] It was subsequently approved by the European Commission at the recommendation of the European Medicines Agency (EMA) in February 2009, for use in adult males with advanced, hormone-dependent prostate cancer.[15] Ferring Pharmaceuticals markets the drug under the name Firmagon.[15]
Research
Degarelix is studied for use as a chemical castration agent on sex offenders in Sweden.[16] A study published on April 29, 2020 in JAMA Psychiatry demonstrated a reduced the risk score for committing child sexual abuse in men with pedophilic disorder 2 weeks after initial injection.[17]
See also
- Gonadotropin-releasing hormone receptor § Antagonists
References
- 1 2 3 4 5 6 7 "Firmagon- degarelix kit". DailyMed. 18 September 2019. Archived from the original on 12 August 2020. Retrieved 26 February 2020.
- 1 2 3 "Degarelix (Firmagon) Use During Pregnancy". Drugs.com. 3 February 2020. Archived from the original on 26 February 2020. Retrieved 25 February 2020.
- ↑ "Firmagon 120mg Injection - Summary of Product Characteristics (SmPC)". (emc). 15 January 2020. Archived from the original on 26 February 2020. Retrieved 25 February 2020.
- 1 2 "Degarelix Monograph for Professionals". Drugs.com. Archived from the original on 26 January 2021. Retrieved 22 December 2021.
- 1 2 3 4 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 992. ISBN 978-0857114105.
- 1 2 "Firmagon". Archived from the original on 26 February 2020. Retrieved 22 December 2021.
- ↑ "Firmagon Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 20 April 2021. Retrieved 22 December 2021.
- 1 2 3 Clinton TN, Woldu SL, Raj GV (June 2017). "Degarelix versus luteinizing hormone-releasing hormone agonists for the treatment of prostate cancer". Expert Opinion on Pharmacotherapy. 18 (8): 825–832. doi:10.1080/14656566.2017.1328056. PMC 7171911. PMID 28480768.
- ↑ Gittelman M, Pommerville PJ, Persson BE, Jensen JK, Olesen TK (November 2008). "A 1-year, open label, randomized phase II dose finding study of degarelix for the treatment of prostate cancer in North America". The Journal of Urology. 180 (5): 1986–92. doi:10.1016/j.juro.2008.07.033. PMID 18801505.
- ↑ Van Poppel H, Tombal B, de la Rosette JJ, Persson BE, Jensen JK, Kold Olesen T (October 2008). "Degarelix: a novel gonadotropin-releasing hormone (GnRH) receptor blocker--results from a 1-yr, multicentre, randomised, phase 2 dosage-finding study in the treatment of prostate cancer". European Urology. 54 (4): 805–13. doi:10.1016/j.eururo.2008.04.065. PMID 18538469.
- ↑ "Firmagon "Ferring Pharmaceuticals A/S" - Felleskatalogen". www.felleskatalogen.no. Archived from the original on 28 November 2020. Retrieved 5 May 2020.
- ↑ van Poppel H, Nilsson S (June 2008). "Testosterone surge: rationale for gonadotropin-releasing hormone blockers?". Urology. 71 (6): 1001–6. doi:10.1016/j.urology.2007.12.070. PMID 18407326.
- ↑ "Drug Approval Package: Degarelix NDA #022201". U.S. Food and Drug Administration (FDA). Archived from the original on 9 August 2020. Retrieved 29 September 2020.
- Lay summary in: (PDF) https://www.accessdata.fda.gov/drugsatfda_docs/nda/2008/022201s000_SumR.pdf.
{{cite web}}: Missing or empty|title=(help)
- Lay summary in: (PDF) https://www.accessdata.fda.gov/drugsatfda_docs/nda/2008/022201s000_SumR.pdf.
- ↑ "FDA Approves Ferring Pharmaceuticals' Degarelix (Generic Name) for Treatment of Advanced Prostate Cancer" (Press release). Ferring Pharmaceuticals. 24 December 2008. Archived from the original on 8 June 2011. Retrieved 25 February 2020 – via PR Newswire.
- 1 2 "Firmagon EPAR". European Medicines Agency (EMA). 10 January 2020. Archived from the original on 26 February 2020. Retrieved 25 February 2020.
- ↑ "Pedofiler ska stoppas – med kemisk kastrering". Expressen. Archived from the original on 26 February 2021. Retrieved 26 December 2020.
- ↑ Landgren V, Malki K, Bottai M, Arver S, Rahm C (April 2020). "Effect of Gonadotropin-Releasing Hormone Antagonist on Risk of Committing Child Sexual Abuse in Men With Pedophilic Disorder: A Randomized Clinical Trial". JAMA Psychiatry. 77 (9): 897–905. doi:10.1001/jamapsychiatry.2020.0440. PMC 7191435. PMID 32347899.
External links
| External sites: |
|
|---|---|
| Identifiers: |