Ganirelix
 | |
| Names | |
|---|---|
| Trade names | Orgalutran, Antagon, Fyremadel, others |
IUPAC name
| |
| Clinical data | |
| Drug class | GnRH antagonist |
| Main uses | Preventing early ovulation[1] |
| Side effects | Abdominal pain, headache, vaginal bleeding, nausea, ovarian hyperstimulation syndrome[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Subcutaneous injection |
| External links | |
| AHFS/Drugs.com | Monograph |
| Legal | |
| License data | |
| Legal status |
|
| Pharmacokinetics | |
| Bioavailability | 91.1% |
| Protein binding | 81.9% |
| Elimination half-life | 16.2 hours |
| Excretion | Feces: 75% Urine: 22% |
| Chemical and physical data | |
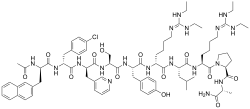
| Formula | C80H113ClN18O13 |
| Molar mass | 1570.35 g·mol−1 |
InChI
| |
Ganirelix, sold under the brand names Orgalutran among others, is a medication used in assisted reproduction to prevent early ovulation.[1] This can increase the number of suitable eggs available for in-vitro fertilisation.[1] It is given by injection under the skin.[3]
Common side effects include abdominal pain, headache, vaginal bleeding, nausea, and ovarian hyperstimulation syndrome.[2] It should not be used in those with latex allergies.[4] It should not be used once pregnancy has occurred.[2] It is a gonadotropin-releasing hormone (GnRH) antagonist.[3] It works by blocking receptors for GnRH, which decreases LH and therefore stops ovulation until this is desired.[1]
Ganirelix was approved for medical use in the United States in 1999 and Europe in 2000.[2][1] In the United States 5 doses of 0.25 mg costs about 380 USD as of 2021.[5]
Medical uses
Ganirelix is used as part of fertility treatment in those with intact ovaries. Specifically, it is used to prevent premature ovulation in those undergoing fertility treatment involving ovarian hyperstimulation that causes the ovaries to produce multiple eggs. When such premature ovulation occurs, the eggs released by the ovaries may be too immature to be used in in-vitro fertilization. Ganirelix prevents ovulation until it is triggered by injecting human chorionic gonadotrophin (hCG).[6]
Dosage
Ganirelix is administered by a subcutaneous injection of 250 µg once per day during the mid to late follicular phase of a person's menstrual cycle. Treatment should start on the 5th or 6th day after the start of ovarian stimulation, and the mean duration for its use is five days.[6] Preferably, the subcutaneous injections are delivered in the upper leg, and the person can be trained to do this themself. Continued use of the drug should take place until the administration of hCG begins. hCG administration is begun when a sufficient number of follicles have developed due to the effects of endogenous and or exogenously administered follicle stimulating hormone.[6]
Contraindications
Ganirelix should not be used in women who are already pregnant, and because of this the onset of pregnancy must be ruled out before it is administered. Women using ganirelix should not breast feed, as it is not known whether ganirelix is excreted in breast milk.[6]
Side effects
Clinical studies have shown that the most common side effect is a slight reaction at the site of injection in the form of redness, and sometimes swelling.[6] Clinical studies have shown that, one hour after injection, the incidence of at least one moderate or severe local skin reaction per treatment cycle was 12% in 4 patients treated with ganirelix and 25% in patients treated subcutaneously with a GnRH agonist. The local reactions generally disappear within 4 hours after administration.[6] Other reported side effects are some that are known to be associated with ovarian hyperstimulation, including gynecological abdominal pain, headache, vaginal bleeding, nausea, and gastrointestinal abdominal pain. In some rare cases, less than 1 user in 10,000, hypersensitivity to ganirelix can cause anaphylactoid reactions, most likely due to allergy.[7]
Birth defects
A follow-up analysis for ganirelix done by the Marketing Authorisation Holder compared the number of congenital malformations between individuals whose mothers were treated with ganirelix compared with individuals whose mothers were treated with a GnRH agonist. The total number of congenital malformations was higher in the ganirelix group than in the GnRH agonist group (7.6% vs. 5.5%).[8] This falls within the range for the normal incidence of congenital malformations, and current data do not suggest that ganirelix increases the incidence of congenital malformations or anomalies. No important differences in the frequency of ectopic pregnancies and miscarriage were noted with the use of ganirelix.[8]
Interactions
Currently, no studies have been done to assess the possible drug-drug interactions between ganirelix and other drugs.[8]
Pharmacology
Pharmacodynamics
Ganierlix is a synthetic peptide that works as an antagonist against gonadotropin-releasing hormone (GnRH) ("Ganirelix acetate injection," 2009). Ganirelix competitively blocks GnRH receptors on the pituitary gonadotroph, quickly resulting in the suppression of gonadotropin secretion.[7] This suppression is easily reversed by discontinuation of ganirelix administration. Ganirelix has a significantly higher receptor binding affinity (Kd = 0.4 nM) than GnRH (Kd = 3.6 nM).[6]
Pharmacokinetics
When ganirelix is given to healthy adult females, steady-state serum concentrations are reached, on average, after three days ("Ganirelix acetate injection," 2009). A study administering ganirelix to healthy adult females (n=15) found the mean (SD) elimination half-life (t1/2) to be 16.2(1.6) hours, volume of distribution/absolute bioavailability (Vd/F) 76.5(10.3) liters, maximum serum concentration (Cmax) 11.2(2.4) ng/mL, and the time until maximum concentration (tmax) 1.1(0.2) hours. One 250 µg injection of ganirelix resulted in a mean absolute bioavailability of 91.1%.[7]
Chemistry
Ganirelix is derived from GnRH, with amino acid substitutions made at positions 1, 2, 3, 6, 8, and 10.[7]
History
The European Commission gave marketing authorization for ganirelix throughout the European Union to N.V. Organon in May 2000.[6]
See also
- Gonadotropin-releasing hormone receptor § Antagonists
References
- 1 2 3 4 5 "Orgalutran". Archived from the original on 12 November 2020. Retrieved 3 December 2021.
- 1 2 3 4 "Ganirelix Monograph for Professionals". Drugs.com. Archived from the original on 21 January 2021. Retrieved 3 December 2021.
- 1 2 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 778. ISBN 978-0857114105.
- ↑ Canada, Health (29 August 2018). "Health Product InfoWatch - September 2018". www.canada.ca. Archived from the original on 3 November 2021. Retrieved 3 December 2021.
- ↑ "Ganirelix Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 23 April 2017. Retrieved 3 December 2021.
- 1 2 3 4 5 6 7 8 "Orgalutran". European Medicines Agency. Archived from the original on 9 October 2014. Retrieved 11 May 2012.
- 1 2 3 4 Organon Pharmaceuticals USA. "Ganirelix Acetate Injection". DailyMed. Archived from the original on 5 March 2016. Retrieved 11 May 2012.
- 1 2 3 "Orgalutran : EPAR – Scientific Discussion". European Medicines Agency. Archived from the original on 6 May 2014. Retrieved 13 May 2012.
External links
| External sites: |
|
|---|---|
| Identifiers: |
- "Ganirelix acetate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 2021-10-31. Retrieved 2021-08-05.