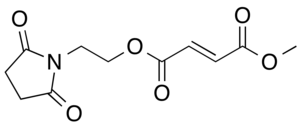
Diroximel fumarate
 | |
| Names | |
|---|---|
| Trade names | Vumerity |
| Other names | ALKS-8700 |
| Clinical data | |
| Main uses | Relapsing forms of multiple sclerosis (MS)[1] |
| Side effects | Flushing, abdominal pain, diarrhea, nausea[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Routes of use | By mouth |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620002 |
| Legal | |
| License data | |
| Legal status | |
| Chemical and physical data | |
| Formula | C11H13NO6 |
| Molar mass | 255.226 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Diroximel fumarate, sold under the brand name Vumerity, is a medication used to treat relapsing forms of multiple sclerosis (MS).[1] This includes clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease.[1] It is taken by mouth.[1]
Common side effects include flushing, abdominal pain, diarrhea, and nausea.[1] Other side effects may include anaphylaxis, progressive multifocal leukoencephalopathy, herpes zoster, liver problems, and low lymphocytes.[1] Use in pregnancy may harm the baby.[2] It works after the body converts it into monomethyl fumarate (MMF).[3]
Diroximel fumarate was approved for medical use in the United States in 2019.[3] While it was approved in Europe and the United Kingdom in 2021 it is not yet commercially available.[3] In the United States it costs about 7,900 USD per day as of 2021.[4]
Medical uses
It is used for the treatment of relapsing forms of multiple sclerosis (MS).[1][5][6]
Dosage
It is started at 231 mg twice per day for a week.[1] It is than taken as 462 mg twice per day.[1]
Society and culture
This drug was formulated by Alkermes in collaboration with Biogen.[7]
References
- 1 2 3 4 5 6 7 8 9 10 11 "Vumerity- diroximel fumarate capsule". DailyMed. Archived from the original on 5 February 2021. Retrieved 1 February 2021.
- ↑ "Diroximel fumarate Pregnancy and Breastfeeding Warnings". Archived from the original on 14 February 2021. Retrieved 26 December 2021.
- 1 2 3 "Vumerity · Relapsing remitting multiple sclerosis (MS)". Archived from the original on 19 May 2021. Retrieved 26 December 2021.
- ↑ "Vumerity Prices, Coupons and Patient Assistance Programs". Retrieved 26 December 2021.
- ↑ Wang Y, Bhargava P (July 2020). "Diroximel fumarate to treat multiple sclerosis". Drugs of Today (Barcelona, Spain : 1998). 56 (7): 431–437. doi:10.1358/dot.2020.56.7.3151521. PMID 32648853.
- ↑ Kourakis S, Timpani CA, de Haan JB, Gueven N, Fischer D, Rybalka E (October 2020). "Dimethyl Fumarate and Its Esters: A Drug with Broad Clinical Utility?". Pharmaceuticals (Basel, Switzerland). 13 (10): 306. doi:10.3390/ph13100306. PMC 7602023. PMID 33066228.
- ↑ "Archive copy". Archived from the original on 28 August 2021. Retrieved 30 July 2021.
{{cite web}}: CS1 maint: archived copy as title (link)
External links
| Identifiers: |
|---|
- "Diroximel fumarate". Drug Information Portal. U.S. National Library of Medicine. Archived from the original on 28 August 2021. Retrieved 30 July 2021.