Iron overload
| Iron overload | |
|---|---|
| Other names: Haemochromatosis, hemochromatosis, bronze diabetes[1] | |
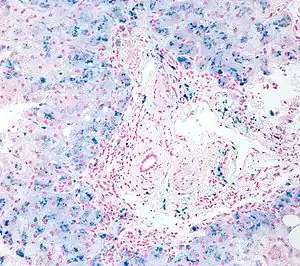 | |
| Micrograph of a liver biopsy showing iron deposits due to iron overload. Iron stain. | |
| Specialty | Hematology, gastroenterology |
| Symptoms | Tiredness, abdominal pain, darkening skin, sexual dysfunction[2] |
| Complications | Cirrhosis, liver failure, liver cancer, heart failure, diabetes, arthritis, infections[2][1] |
| Usual onset | Males > 40, females > 50[2] |
| Types | Primary, secondary[1] |
| Causes | Primary: Hereditary haemochromatosis (HHC)[2] Secondary: Repeated blood transfusions, excessive dietary intake[2] |
| Risk factors | Liver diseases, alcoholism[2] |
| Diagnostic method | Blood tests (high ratio of iron to transferrin), liver biopsy[2] |
| Treatment | Phlebotomy, chelation therapy, liver transplant[2] |
| Frequency | HCC 1 in 400 white people[1] |
Iron overload, also known as hemochromatosis, is the build up of excessive iron in the body.[1] Symptoms may include tiredness, abdominal pain, darkening skin, and sexual dysfunction.[2] Complications may include cirrhosis, liver failure, liver cancer, heart failure, diabetes, arthritis, and infections.[2][1]
The most common causes are hereditary haemochromatosis (HHC), a genetic disorder, and iron overload from repeated blood transfusions.[2] Rarely it may occur due excessive dietary intake or liver injury during early development (the latter known as neonatal hemochromatosis).[2] Risk factors for more severe disease include other liver diseases and alcoholism.[2] Diagnosis is generally by blood tests, which may be supported by a liver biopsy.[2]
Most cases of iron overload due to HCC are treated with the regular removal of blood by phlebotomy.[2] Initially this may be done twice per week and once levels come down may occur two to three times per year.[2] Those who have iron overload due to other reasons may be treated with chelation therapy.[2] Neonatal hemochromatosis may be treated with exchange transfusions and IVIG.[2] Occasionally a liver transplant is required.[2] Iron supplements and vitamin C should be avoided.[2]
About 1 in 400 white people have HHC and it is the most common autosomal recessive disorder in this group.[1] Onset of symptoms in males is usually after the age of 40 and in females after the age of 50.[2] While males and females are equally likely to carry the mutation, males are 2 to 3 times likely to develop complications.[2][1] Outcomes in HHC are good if the disease is detected early and treated.[1] The symptoms of iron overload were first described by Armand Trousseau in 1865, though it was not until 1889 that von Recklinghausen linked the disease to iron.[3]
Signs and symptoms
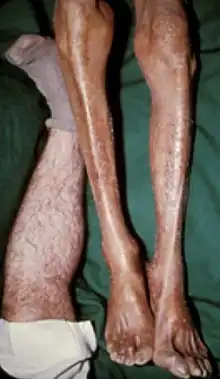
Organs most commonly affected by haemochromatosis include the liver, heart, and endocrine glands.[6]
Iron overload may present with the following syndromes:
- liver: chronic liver disease and cirrhosis of the liver.[7]
- heart: heart failure, irregular heart rhythm.[7]
- hormones: diabetes (see below) and hypogonadism (insufficiency of the sex hormone producing glands) which leads to low sex drive and/or loss of fertility in men and loss of menstrual cycle in women.[7]
- metabolism: diabetes in people with iron overload occurs as a result of selective iron deposition in islet beta cells in the pancreas leading to functional failure and cell death.[8]
- joints: arthritis, from calcium pyrophosphate deposition in joints leading to joint pains. The most commonly affected joints are those of the hands, particularly the knuckles of the second and third fingers.[9]
- skin: melanoderma (darkening or 'bronzing' of the skin).[9][10] The skin's deep tan color, in concert with insulin insufficiency due to pancreatic damage, is the source of a nickname for this condition: "bronze diabetes" (for more information, see the history of haemochromatosis).
Causes
The causes can be distinguished between primary cases (hereditary or genetically determined) and less frequent secondary cases (acquired during life).[11]
Primary
Although it was known most of the 20th century that most cases of haemochromatosis were inherited, they were incorrectly assumed to depend on a single gene.[12] The overwhelming majority depend on mutations of the HFE gene discovered in 1996, but since then others have been discovered and sometimes are grouped together as "non-classical hereditary haemochromatosis",[13] "non-HFE related hereditary haemochromatosis",[14] or "non-HFE haemochromatosis".[15]
| Description | OMIM | Mutation |
| Haemochromatosis type 1: "classical" haemochromatosis | 235200 | HFE |
| Haemochromatosis type 2A: juvenile haemochromatosis | 602390 | Haemojuvelin (HJV, also known as RGMc and HFE2) |
| Haemochromatosis type 2B: juvenile haemochromatosis | 606464 | hepcidin antimicrobial peptide (HAMP) or HFE2B |
| Haemochromatosis type 3 | 604250 | transferrin receptor-2 (TFR2 or HFE3) |
| Haemochromatosis type 4 / African iron overload | 604653 | ferroportin (SLC11A3/SLC40A1) |
| Neonatal haemochromatosis | 231100 | (unknown) |
| Acaeruloplasminaemia (very rare) | 604290 | caeruloplasmin |
| Congenital atransferrinaemia (very rare) | 209300 | transferrin |
| GRACILE syndrome (very rare) | 603358 | BCS1L |
Most types of hereditary haemochromatosis have autosomal recessive inheritance, while type 4 has autosomal dominant inheritance.[16]
Secondary
- Severe chronic haemolysis of any cause, including intravascular haemolysis and ineffective erythropoiesis (haemolysis within the bone marrow)
- Multiple frequent blood transfusions (either whole blood or just red blood cells), which are usually needed either by individuals with hereditary anaemias (such as beta-thalassaemia major, sickle cell anaemia, and Diamond–Blackfan anaemia) or by older patients with severe acquired anaemias such as in myelodysplastic syndromes.[17]
- Excess parenteral iron supplements, such as what can acutely happen in iron poisoning
- Excess dietary iron
- Some disorders do not normally cause haemochromatosis on their own, but may do so in the presence of other predisposing factors. These include cirrhosis (especially related to alcohol abuse), steatohepatitis of any cause, porphyria cutanea tarda, prolonged haemodialysis, and post-portacaval shunting
Diagnosis
_in_pancreatic_islet_beta_cells(red)_from_transfusional_hemochromatosis_autopsy_cases_versus_that_of_controls_(right_lower_one).jpg.webp)
There are several methods available for diagnosing and monitoring iron loading.
Blood tests are usually the first test if there is a clinical suspicion of iron overload. Serum ferritin testing is a low-cost, readily available, and minimally invasive method for assessing body iron stores. However, the major problem with using it as an indicator of iron overload is that it can be elevated in a variety of other medical conditions including infection, inflammation, fever, liver disease, kidney disease, and cancer. Also, total iron binding capacity may be low, but can also be normal.[18] In males and postmenopausal females, normal range of serum ferritin is between 12 and 300 ng/mL (670 pmol/L) .[19][20][21] In premenopausal females, normal range of serum ferritin is between 12 and 150[19] or 200[20] ng/mL (330 or 440 pmol/L).[21] If the person is showing the symptoms, they may need to be tested more than once throughout their lives as a precaution, most commonly in women after menopause. Transferrin saturation is a more specific test.
DNA/screening: the standard of practice in diagnosis of haemochromatosis, places emphasis on genetic testing.[22] Positive HFE analysis confirms the clinical diagnosis of haemochromatosis in asymptomatic individuals with blood tests showing increased iron stores, or for predictive testing of individuals with a family history of haemochromatosis. The alleles evaluated by HFE gene analysis are evident in ~80% of patients with haemochromatosis; a negative report for HFE gene does not rule out haemochromatosis. First degree relatives of those with primary haemochromatosis should be screened to determine if they are a carrier or if they could develop the disease. This can allow preventive measures to be taken. Screening the general population is not recommended.[23]
Liver biopsy is the removal of small sample in order to be studied and can determine the cause of inflammation or cirrhosis. In someone with negative HFE gene testing, elevated iron status for no other obvious reason, and family history of liver disease, additional evaluation of liver iron concentration is indicated. In this case, diagnosis of haemochromatosis is based on biochemical analysis and histologic examination of a liver biopsy. Assessment of the hepatic iron index (HII) is considered the "gold standard" for diagnosis of haemochromatosis.
Magnetic resonance imaging (MRI) is used as a noninvasive way to accurately estimate iron deposition levels in the liver as well as heart, joints, and pituitary gland.
Treatment
Phlebotomy: routine treatment consists of regularly scheduled phlebotomies (bloodletting or erythrocytapheresis). When first diagnosed, the phlebotomies may be performed every week or fortnight, until iron levels can be brought to within normal range. Once the serum ferritin and transferrin saturation are within the normal range, treatments may be scheduled every two to three months depending upon the rate of reabsorption of iron. A phlebotomy session typically draws between 450 and 500 mL of blood.[24] The blood drawn is sometimes donated.[25]
A diet low in iron is generally recommended, but has little effect compared to venesection. The human diet contains iron in two forms: heme iron and non-heme iron. Heme iron is the most easily absorbed form of iron. People with iron overload may be advised to avoid food that are high in heme iron. Highest in heme iron is red meat such as beef, venison, lamb, buffalo, and fish such as bluefin tuna. A strict low-iron diet is usually not necessary. Non-heme iron is not as easily absorbed in the human system and is found in plant-based foods like grains, beans, vegetables, fruits, nuts, and seeds.[26]
Medication: For those unable to tolerate routine blood draws, there are chelating agents available for use.[27] The drug deferoxamine binds with iron in the bloodstream and enhances its elimination in urine and faeces. Typical treatment for chronic iron overload requires subcutaneous injection over a period of 8–12 hours daily. Two newer iron-chelating drugs that are licensed for use in patients receiving regular blood transfusions to treat thalassaemia (and, thus, who develop iron overload as a result) are deferasirox and deferiprone.[28][29]
Chelatation
A novel experimental approach to the hereditary haemochromatosis treatment is the maintenance therapy with polymeric chelators.[30][31][32] These polymers or particles have a negligible or null systemic biological availability and they are designed to form stable complexes with Fe2+ and Fe3+ in the GIT and thus limiting their uptake and long-term accumulation. Although this method has only a limited efficacy, unlike small-molecular chelators, the approach has virtually no side effects in sub-chronic studies.[32] Interestingly, the simultaneous chelation of Fe2+ and Fe3+ increases the treatment efficacy.[32]
Prognosis
In general, provided there has been no liver damage, patients should expect a normal life expectancy if adequately treated by venesection. If the serum ferritin is greater than 1000 ug/L at diagnosis there is a risk of liver damage and cirrhosis which may eventually shorten their life.[33] The presence of cirrhosis increases the risk of hepatocellular carcinoma.[34]
Epidemiology
It is most common in certain European populations (such as the Irish and Norwegians) and occurs in 0.6% of some unspecified population.[23] Men have a 24-fold increased rate of iron-overload disease compared with women.[23]
People of Celtic (Irish, Scottish, Welsh, Cornish, Breton etc.), English, and Scandinavian origin[35] have a particularly high incidence, with about 10% being carriers of the principal genetic variant, the C282Y mutation on the HFE gene, and 1% having the condition.[36] This has been recognised in several alternative names such as Celtic curse, Irish illness, British gene, and Scottish sickness.
History
Stone Age
Diet and the environment are thought to have had large influence on the mutation of genes related to iron overload. Starting during the Mesolithic era, communities of people lived in an environment that was fairly sunny, warm and had the dry climates of the Middle East. Most humans who lived at that time were foragers and their diets consisted largely of game, fish and wild plants. Archaeologists studying dental plaque have found evidence of tubers, nuts, plantains, grasses and other foods rich in iron. Over many generations, the human body became well-adapted to a high level of iron content in the diet.[37]
In the Neolithic era, significant changes are thought to have occurred in both the environment and diet. Some communities of foragers migrated north, leading to changes in lifestyle and environment, with a decrease in temperatures and a change in the landscape which the foragers then needed to adapt to. As people began to develop and advance their tools, they learned new ways of producing food, and farming also slowly developed. These changes would have led to serious stress on the body and a decrease in the consumption of iron-rich foods. This transition is a key factor in the mutation of genes, especially those that regulated dietary iron absorption. Iron, which makes up 70% of red blood cell composition, is a critical micronutrient for effective thermoregulation in the body.[38] Iron deficiency will lead to a drop in the core temperature. In the chilly and damp environments of Northern Europe, supplementary iron from food was necessary to keep temperatures regulated, however, without sufficient iron intake the human body would have started to store iron at higher rates than normal. In theory, the pressures caused by migrating north would have selected for a gene mutation that promoted greater absorption and storage of iron.[39]
Viking hypothesis
Studies and surveys conducted to determine the frequencies of hemochromatosis help explain how the mutation migrated around the globe. In theory, the disease initially evolved from travelers migrating from the north. Surveys show a particular distribution pattern with large clusters and frequencies of gene mutations along the western European coastline.[40] This led the development of the "Viking Hypothesis".[41] Cluster locations and mapped patterns of this mutation correlate closely to the locations of Viking settlements in Europe established c.700 AD to c.1100 AD. The Vikings originally came from Norway, Sweden and Denmark. Viking ships made their way along the coastline of Europe in search of trade, riches, and land. Genetic studies suggest that the extremely high frequency patterns in some European countries are the result of migrations of Vikings and later Normans, indicating a genetic link between hereditary hemochromatosis and Viking ancestry.[42]
Modern times
In 1865, Armand Trousseau (a French internist) was one of the first to describe many of the symptoms of a diabetic person with cirrhosis of the liver and bronzed skin color. The term hemochromatosis was first used by German pathologist Friedrich Daniel von Recklinghausen in 1889 when he described an accumulation of iron in body tissues.[43] In 1935 J.H. Sheldon, a British physician, described the link to iron metabolism for the first time as well as demonstrating its hereditary nature.[43]
In 1996 Felder and colleagues identified the hemochromatosis gene, HFE gene. Felder found that the HFE gene has two main mutations, C282Y and H63D, which were the main cause of hereditary hemochromatosis.[44] The next year the CDC and the National Human Genome Research Institute sponsored an examination of hemochromatosis following the discovery of the HFE gene, which helped lead to the population screenings and estimates that are still being used today.[45]
Terminology
Historically, the term haemochromatosis (spelled hemochromatosis in American English) was initially used to refer to what is now more specifically called haemochromatosis type 1 (or HFE-related hereditary haemochromatosis). Currently, haemochromatosis (without further specification) is mostly defined as iron overload with a hereditary or primary cause,[46][47] or originating from a metabolic disorder.[48] However, the term is currently also used more broadly to refer to any form of iron overload, thus requiring specification of the cause, for example, hereditary haemochromatosis. Hereditary haemochromatosis is an autosomal recessive disorder with estimated prevalence in the population of 1 in 200 among patients with European ancestry, with lower incidence in other ethnic groups.[49] The gene responsible for hereditary haemochromatosis (known as HFE gene) is located on chromosome 6; the majority of hereditary haemochromatosis patients have mutations in this HFE gene.
Hereditary haemochromatosis is characterized by an accelerated rate of intestinal iron absorption and progressive iron deposition in various tissues. This typically begins to be expressed in the third to fifth decades of life, but may occur in children. The most common presentation is hepatic (liver) cirrhosis in combination with hypopituitarism, cardiomyopathy, diabetes, arthritis, or hyperpigmentation. Because of the severe sequelae of this disorder if left untreated, and recognizing that treatment is relatively simple, early diagnosis before symptoms or signs appear is important.[22][50]
In general, the term haemosiderosis is used to indicate the pathological effect of iron accumulation in any given organ, which mainly occurs in the form of the iron-storage complex haemosiderin.[51][52] Sometimes, the simpler term siderosis is used instead.
Other definitions distinguishing haemochromatosis or haemosiderosis that are occasionally used include:
- Haemosiderosis is haemochromatosis caused by excessive blood transfusions, that is, haemosiderosis is a form of secondary haemochromatosis.[53][54]
- Haemosiderosis is haemosiderin deposition within cells, while haemochromatosis is haemosiderin within cells and interstitium.[55]
- Haemosiderosis is iron overload that does not cause tissue damage,[56] while haemochromatosis does.[57]
- Haemosiderosis is arbitrarily differentiated from haemochromatosis by the reversible nature of the iron accumulation in the reticuloendothelial system.[58]
References
- 1 2 3 4 5 6 7 8 9 Porter, JL; Rawla, P (January 2020). "Hemochromatosis". PMID 28613612.
{{cite journal}}: Cite journal requires|journal=(help) - 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 "Hemochromatosis". National Institute of Diabetes and Digestive and Kidney Diseases. NIDDK. Archived from the original on 22 March 2021. Retrieved 24 February 2021.
- ↑ Barton, James C.; Edwards, Corwin Q. (2000). Hemochromatosis: Genetics, Pathophysiology, Diagnosis and Treatment. Cambridge University Press. p. 3. ISBN 978-0-521-59380-9. Archived from the original on 2021-08-28. Retrieved 2021-02-24.
- ↑ Herbert, Fred (2007). Images of Memorable Cases: 50 Years at the Bedside. p. Case 148. Archived from the original on 28 August 2021. Retrieved 24 February 2021.
- ↑ Raju, K; Venkataramappa, SM (January 2018). "Primary Hemochromatosis Presenting as Type 2 Diabetes Mellitus: A Case Report with Review of Literature". International journal of applied & basic medical research. 8 (1): 57–60. doi:10.4103/ijabmr.IJABMR_402_16. PMID 29552540.
- ↑ Andrews, Nancy C. (1999). "Disorders of Iron Metabolism". New England Journal of Medicine. 341 (26): 1986–95. doi:10.1056/NEJM199912233412607. PMID 10607817.
- 1 2 3 John Murtagh (2007). General Practice. McGraw Hill Australia. ISBN 978-0-07-470436-3.
- ↑ Lu, JP (1994). "Selective iron deposition in pancreatic islet B cells of transfusional iron‐overloaded autopsy cases". Pathol Int. 44: 194–199. doi:10.1111/j.1440-1827.1994.tb02592.x. PMID 8025661.
- 1 2 Bruce R Bacon, Stanley L Schrier. "Patient information: Hemochromatosis (hereditary iron overload) (Beyond the Basics)". UpToDate. Archived from the original on 2016-06-19. Retrieved 2016-07-14. Literature review current through: Jun 2016. | This topic last updated: Apr 14, 2015.
- ↑ Brissot, P; Pietrangelo, A; Adams, PC; de Graaff, B; McLaren, CE; Loréal, O (5 April 2018). "Haemochromatosis". Nature reviews. Disease primers. 4: 18016. doi:10.1038/nrdp.2018.16. PMC 7775623. PMID 29620054.
- ↑ Pietrangelo, A (2003). "Haemochromatosis". Gut. 52 (90002): ii23–30. doi:10.1136/gut.52.suppl_2.ii23. PMC 1867747. PMID 12651879.
- ↑ Cam Patterson; Marschall S. Runge (2006). Principles of molecular medicine. Totowa, NJ: Humana Press. p. 567. ISBN 978-1-58829-202-5.
- ↑ Mendes, Ana Isabel; Ferro, Ana; Martins, Rute; Picanço, Isabel; Gomes, Susana; Cerqueira, Rute; Correia, Manuel; Nunes, António Robalo; Esteves, Jorge; Fleming, Rita; Faustino, Paula (2008). "Non-classical hereditary hemochromatosis in Portugal: novel mutations identified in iron metabolism-related genes" (PDF). Annals of Hematology. 88 (3): 229–34. doi:10.1007/s00277-008-0572-y. PMID 18762941. Archived (PDF) from the original on 2019-08-29. Retrieved 2019-07-05.
- ↑ Maddrey, Willis C.; Schiff, Eugene R.; Sorrell, Michael F. (2007). Schiff's diseases of the liver. Hagerstwon, MD: Lippincott Williams & Wilkins. p. 1048. ISBN 978-0-7817-6040-9.
- ↑ Pietrangelo, Antonello (2005). "Non-HFE Hemochromatosis". Seminars in Liver Disease. 25 (4): 450–60. doi:10.1055/s-2005-923316. PMID 16315138.
- ↑ Franchini, Massimo (2006). "Hereditary iron overload: Update on pathophysiology, diagnosis, and treatment". American Journal of Hematology. 81 (3): 202–9. doi:10.1002/ajh.20493. PMID 16493621.
- ↑ Lu, JP (1994). "Selective iron deposition in pancreatic islet B cells of transfusional iron‐overloaded autopsy cases". Pathol Int. 44: 194–199. doi:10.1111/j.1440-1827.1994.tb02592.x. PMID 8025661.
- ↑ labtestsonline.org TIBC & UIBC, Transferrin Archived 2010-11-25 at the Wayback Machine Last reviewed on October 28, 2009.
- 1 2 Ferritin Archived 2016-07-05 at the Wayback Machine by: Mark Levin, MD, Hematologist and Oncologist, Newark, NJ. Review provided by VeriMed Healthcare Network
- 1 2 Andrea Duchini. "Hemochromatosis Workup". Medscape. Archived from the original on 2017-11-07. Retrieved 2016-07-14. Updated: Jan 02, 2016
- 1 2 Molar concentration is derived from mass value using molar mass of 450,000 g•mol−1 for ferritin
- 1 2 Pietrangelo, Antonello (2010). "Hereditary Hemochromatosis: Pathogenesis, Diagnosis, and Treatment". Gastroenterology. 139 (2): 393–408. doi:10.1053/j.gastro.2010.06.013. PMID 20542038.
- 1 2 3 Crownover, BK; Covey, CJ (Feb 1, 2013). "Hereditary hemochromatosis". American Family Physician. 87 (3): 183–90. PMID 23418762.
- ↑ Barton, James C. (1 December 1998). "Management of Hemochromatosis". Annals of Internal Medicine. 129 (11_Part_2): 932–9. doi:10.7326/0003-4819-129-11_Part_2-199812011-00003. PMID 9867745.
- ↑ NIH blood bank. "Hemochromatosis Donor Program". Archived from the original on 2018-10-22. Retrieved 2019-02-22.
- ↑ "Welcome". Hemochromatosis.org - An Education Website for Hemochromatosis and Too Much Iron. Archived from the original on 2018-04-11. Retrieved 2018-04-11.
- ↑ Miller, Marvin J. (1989-11-01). "Syntheses and therapeutic potential of hydroxamic acid based siderophores and analogs". Chemical Reviews. 89 (7): 1563–1579. doi:10.1021/cr00097a011.
- ↑ Choudhry VP, Naithani R (2007). "Current status of iron overload and chelation with deferasirox". Indian J Pediatr. 74 (8): 759–64. doi:10.1007/s12098-007-0134-7. PMID 17785900.
- ↑ Hoffbrand, A. V. (20 March 2003). "Role of deferiprone in chelation therapy for transfusional iron overload". Blood. 102 (1): 17–24. doi:10.1182/blood-2002-06-1867. PMID 12637334.
- ↑ Polomoscanik, Steven C.; Cannon, C. Pat; Neenan, Thomas X.; Holmes-Farley, S. Randall; Mandeville, W. Harry; Dhal, Pradeep K. (2005). "Hydroxamic Acid-Containing Hydrogels for Nonabsorbed Iron Chelation Therapy: Synthesis, Characterization, and Biological Evaluation". Biomacromolecules. 6 (6): 2946–2953. doi:10.1021/bm050036p. ISSN 1525-7797.
- ↑ Qian, Jian; Sullivan, Bradley P.; Peterson, Samuel J.; Berkland, Cory (2017). "Nonabsorbable Iron Binding Polymers Prevent Dietary Iron Absorption for the Treatment of Iron Overload". ACS Macro Letters. 6 (4): 350–353. doi:10.1021/acsmacrolett.6b00945. ISSN 2161-1653.
- 1 2 3 Groborz, Ondřej; Poláková, Lenka; Kolouchová, Kristýna; Švec, Pavel; Loukotová, Lenka; Miriyala, Vijay Madhav; Francová, Pavla; Kučka, Jan; Krijt, Jan; Páral, Petr; Báječný, Martin; Heizer, Tomáš; Pohl, Radek; Dunlop, David; Czernek, Jiří; Šefc, Luděk; Beneš, Jiří; Štěpánek, Petr; Hobza, Pavel; Hrubý, Martin (2020). "Chelating Polymers for Hereditary Hemochromatosis Treatment". Macromolecular Bioscience: 2000254. doi:10.1002/mabi.202000254. ISSN 1616-5187.
- ↑ Allen, KJ; Gurrin, LC; Constantine, CC; Osborne, NJ; Delatycki, MB; Nicoll, AJ; McLaren, CE; Bahlo, M; Nisselle, AE; Vulpe, CD; Anderson, GJ; Southey, MC; Giles, GG; English, DR; Hopper, JL; Olynyk, JK; Powell, LW; Gertig, DM (17 January 2008). "Iron-overload-related disease in HFE hereditary hemochromatosis" (PDF). The New England Journal of Medicine. 358 (3): 221–30. doi:10.1056/NEJMoa073286. PMID 18199861. Archived (PDF) from the original on 28 August 2021. Retrieved 23 February 2019.
- ↑ Kowdley, KV (November 2004). "Iron, hemochromatosis, and hepatocellular carcinoma". Gastroenterology. 127 (5 Suppl 1): S79–86. doi:10.1016/j.gastro.2004.09.019. PMID 15508107.
- ↑ The Atlantic: "The Iron in Our Blood That Keeps and Kills Us" by Bradley Wertheim Archived 2017-04-18 at the Wayback Machine January 10, 2013
- ↑ "Hemachromatosis". Encyclopædia Britannica.com. Archived from the original on 18 April 2017. Retrieved 17 April 2017.
- ↑ "The Evolution of Diet". National Geographic. Archived from the original on 2014-08-18. Retrieved 2018-04-11.
- ↑ Rosenzweig, P. H.; Volpe, S. L. (March 1999). "Iron, thermoregulation, and metabolic rate". Critical Reviews in Food Science and Nutrition. 39 (2): 131–148. doi:10.1080/10408399908500491. ISSN 1040-8398. PMID 10198751.
- ↑ Heath, Kathleen M.; Axton, Jacob H.; McCullough, John M.; Harris, Nathan (May 2016). "The evolutionary adaptation of the C282Y mutation to culture and climate during the European Neolithic". American Journal of Physical Anthropology. 160 (1): 86–101. doi:10.1002/ajpa.22937. ISSN 0002-9483. PMC 5066702. PMID 26799452.
- ↑ "Clinical Penetrance of HFE Hereditary Hemochromatosis, Serum Ferritin Levels, and Screening Implications: Can We Iron This Out?". www.hematology.org. 2008-05-01. Archived from the original on 2018-06-15. Retrieved 2018-04-11.
- ↑ Symonette, Caitlin J; Adams, Paul C (June 2011). "Do all hemochromatosis patients have the same origin? A pilot study of mitochondrial DNA and Y-DNA". Canadian Journal of Gastroenterology. 25 (6): 324–326. doi:10.1155/2011/463810. ISSN 0835-7900. PMC 3142605. PMID 21766093.
- ↑ "Videos: Hereditary Hemochromatosis | Canadian Hemochromatosis Society". www.toomuchiron.ca. Archived from the original on 2018-04-11. Retrieved 2018-04-11.
- 1 2 Fitzsimons, Edward J.; Cullis, Jonathan O.; Thomas, Derrick W.; Tsochatzis, Emmanouil; Griffiths, William J. H.; the British Society for Haematology (May 2018). "Diagnosis and therapy of genetic haemochromatosis (review and 2017 update)". British Journal of Haematology. 181 (3): 293–303. doi:10.1111/bjh.15164. PMID 29663319.
- ↑ Feder, J.N.; Gnirke, A.; Thomas, W.; Tsuchihashi, Z.; Ruddy, D.A.; Basava, A.; Dormishian, F.; Domingo, R.; Ellis, M.C. (August 1996). "A novel MHC class I–like gene is mutated in patients with hereditary haemochromatosis". Nature Genetics. 13 (4): 399–408. doi:10.1038/ng0896-399. PMID 8696333.
- ↑ Burke, Wylie; Thomson, Elizabeth; Khoury, Muin J.; McDonnell, Sharon M.; Press, Nancy; Adams, Paul C.; Barton, James C.; Beutler, Ernest; Brittenham, Gary (1998-07-08). "Hereditary Hemochromatosis: Gene Discovery and Its Implications for Population-Based Screening". JAMA. 280 (2): 172–8. doi:10.1001/jama.280.2.172. ISSN 0098-7484. PMID 9669792.
- ↑ thefreedictionary.com > hemochromatosis Archived 2011-06-12 at the Wayback Machine, citing:
- The American Heritage Medical Dictionary, 2004 by Houghton Mifflin Company
- McGraw-Hill Concise Dictionary of Modern Medicine. 2002
- ↑ Merriam-Webster's Medical Dictionary > hemochromatosis Archived 2010-02-21 at the Wayback Machine Retrieved on December 11, 2009
- ↑ thefreedictionary.com Archived 2011-06-12 at the Wayback Machine, citing:
- Dorland's Medical Dictionary for Health Consumers, 2007
- Mosby's Medical Dictionary, 8th edition. 2009
- Jonas: Mosby's Dictionary of Complementary and Alternative Medicine. 2005.
- ↑ "Hemochromatosis". Archived from the original on 2007-03-18. Retrieved 2012-10-05.
- ↑ Brandhagen, D J; Fairbanks, V F; Batts, K P; Thibodeau, S N (1999). "Update on hereditary hemochromatosis and the HFE gene". Mayo Clinic Proceedings. 74 (9): 917–21. doi:10.4065/74.9.917. PMID 10488796.
- ↑ Merriam-Webster's Medical Dictionary > hemosideroses Archived 2010-02-21 at the Wayback Machine Retrieved on December 11, 2009
- ↑ thefreedictionary.com > hemosiderosis Archived 2008-07-04 at the Wayback Machine, citing:
- The American Heritage Medical Dictionary, 2004 by Houghton Mifflin Company
- Mosby's Medical Dictionary, 8th edition.
- ↑ eMedicine Specialties > Radiology > Gastrointestinal > Hemochromatosis Archived 2009-12-12 at the Wayback Machine Author: Sandor Joffe, MD. Updated: May 8, 2009
- ↑ thefreedictionary.com > hemosiderosis Archived 2008-07-04 at the Wayback Machine, citing:
- Gale Encyclopedia of Medicine. Copyright 2008
- ↑ Notecards on radiology gamuts, diseases, anatomy Archived 2010-07-21 at the Wayback Machine 2002, Charles E. Kahn, Jr., MD. Medical College of Wisconsin
- ↑ thefreedictionary.com > hemosiderosis Archived 2008-07-04 at the Wayback Machine, citing:
- Dorland's Medical Dictionary for Health Consumers, 2007
- Mosby's Dental Dictionary, 2nd edition.
- Saunders Comprehensive Veterinary Dictionary, 3rd ed. 2007
- ↑ The HealthScout Network > Health Encyclopedia > Diseases and Conditions > Hemochromatosis Archived 2010-02-09 at the Wayback Machine Retrieved on December 11, 2009
- ↑ thefreedictionary.com > hemosiderosis Archived 2008-07-04 at the Wayback Machine, citing:
- McGraw-Hill Concise Dictionary of Modern Medicine. 2002
External links
| Classification | |
|---|---|
| External resources |
- Iron overload at Curlie
- GeneReview/NCBI/NIH/UW entry on HFE-Associated Hereditary Hemochromatosis Archived 2010-05-27 at the Wayback Machine
- GeneReview/NCBI/NIH/UW entry on TFR2-Related Hereditary Hemochromatosis Archived 2010-06-02 at the Wayback Machine
- GeneReview/NCBI/NIH/UW entry on Juvenile Hereditary Hemochromatosis Archived 2010-06-02 at the Wayback Machine
- GeneReview/NCBI/NIH/UW entry on Aceruloplasminemia Archived 2010-04-10 at the Wayback Machine