Interferon gamma release assay
| Interferon gamma release assay | |
|---|---|
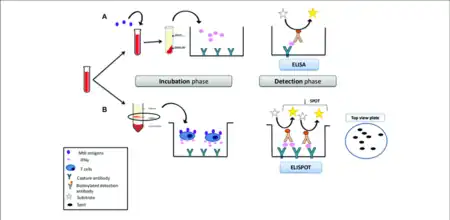 a) Mycobacterium tuberculosis IFN-γ-release assay b) the ELISPOT method[1] | |
| Purpose | Diagnosis of infectious disease(Tuberculosis) |
Interferon-γ release assays (IGRA) are medical tests used in the diagnosis of some infectious diseases, especially tuberculosis. Interferon-γ (IFN-γ) release assays rely on the fact that T-lymphocytes will release IFN-γ when exposed to specific antigens. These tests are mostly developed for the field of tuberculosis diagnosis, but in theory, may be used in the diagnosis of other diseases which rely on cell-mediated immunity, e.g. cytomegalovirus and leishmaniasis and COVID-19.[2] For example, in patients with cutaneous adverse drug reactions, challenge of peripheral blood lymphocytes with the drug causing the reaction produced a positive test result for half of the drugs tested.[3]
There are currently two IFN-γ release assays available for the diagnosis of tuberculosis:
- QuantiFERON-TB Gold (licensed in US, Europe and Japan); and
- T-SPOT.TB, a form of ELISpot, the variant of ELISA (licensed in Europe, US, Japan and China).[4]
The former test quantitates the amount of IFN-γ produced in response to the ESAT-6 and CFP-10 antigens from Mycobacterium tuberculosis, which are distinguishable from those present in BCG and most other non-tuberculous mycobacteria. The latter test determines the total number of individual effector T cells expressing IFN-γ.
The indications for the test are still disputed. It has been evaluated for the diagnosis of latent tuberculosis in HIV patients (who frequently have a negative Mantoux test).[5]
References
- ↑ Albert-Vega, Chloé; Tawfik, Dina M.; Trouillet-Assant, Sophie; Vachot, Laurence; Mallet, François; Textoris, Julien (2018). "Immune Functional Assays, From Custom to Standardized Tests for Precision Medicine". Frontiers in Immunology. 9. doi:10.3389/fimmu.2018.02367/full. ISSN 1664-3224. Archived from the original on 2022-06-15. Retrieved 2023-11-15.
- ↑ Murugesan K, Jagannathan P, Pham TD, Pandey S, Bonilla HF, Jacobson K, Parsonnet J, Andrews JR, Weiskopf D, Sette A, Pinsky BA, Singh U, Banaei N. Interferon-γ Release Assay for Accurate Detection of Severe Acute Respiratory Syndrome Coronavirus 2 T-Cell Response. Clin Infect Dis. 2021 Nov 2;73(9):e3130-e3132. doi: 10.1093/cid/ciaa1537. PMID: 33035306; PMCID: PMC7665338.
- ↑ Halevy, S.; Cohen, A.; Grossman, N. (2005). "Clinical implications of in vitro drug-induced interferon gamma release from peripheral blood lymphocytes in cutaneous adverse drug reactions". Journal of the American Academy of Dermatology. 52 (2): 254–261. doi:10.1016/j.jaad.2004.05.006. PMID 15692470.
- ↑ "How the T-SPOT.TB Test Works". Archived from the original on 2020-02-04. Retrieved 2023-10-20.
- ↑ Lawn SD, Bangani N, Vogt M, et al. (2007). "Utility of interferon-γ ELISPOT assay responses in highly tuberculosis-exposed patients with advanced HIV infection in South Africa". BMC Infectious Diseases. 7: 99. doi:10.1186/1471-2334-7-99. PMC 2031899. PMID 17725839.
