Enzyme inhibitor

An enzyme inhibitor is a molecule that binds to an enzyme and decreases its activity. By binding to enzymes' active sites, inhibitors reduce the compatibility of substrate and enzyme and this leads to the inhibition of Enzyme-Substrate complexes' formation, preventing the catalysis of reactions and decreasing (at times to zero) the amount of product produced by a reaction. It can be said that as the concentration of enzyme inhibitors increases, the rate of enzyme activity decreases, and thus, the amount of product produced is inversely proportional to the concentration of inhibitor molecules. Since blocking an enzyme's activity can kill a pathogen or correct a metabolic imbalance, many drugs are enzyme inhibitors. They are also used in pesticides. Not all molecules that bind to enzymes are inhibitors; enzyme activators bind to enzymes and increase their enzymatic activity, while enzyme substrates bind and are converted to products in the normal catalytic cycle of the enzyme.
The binding of an inhibitor can stop a substrate from entering the enzyme's active site and/or hinder the enzyme from catalyzing its reaction. Inhibitor binding is either reversible or irreversible. Irreversible inhibitors usually react with the enzyme and change it chemically (e.g. via covalent bond formation). These inhibitors modify key amino acid residues needed for enzymatic activity. In contrast, reversible inhibitors bind non-covalently and different types of inhibition are produced depending on whether these inhibitors bind to the enzyme, the enzyme-substrate complex, or both.
Many drug molecules are enzyme inhibitors, so their discovery and improvement is an active area of research in biochemistry and pharmacology.[1] A medicinal enzyme inhibitor is often judged by its specificity (its lack of binding to other proteins) and its potency (its dissociation constant, which indicates the concentration needed to inhibit the enzyme). A high specificity and potency ensure that a drug will have few side effects and thus low toxicity.
Enzyme inhibitors also occur naturally and are involved in the regulation of metabolism. For example, enzymes in a metabolic pathway can be inhibited by downstream products. This type of negative feedback slows the production line when products begin to build up and is an important way to maintain homeostasis in a cell. Other cellular enzyme inhibitors are proteins that specifically bind to and inhibit an enzyme target. This can help control enzymes that may be damaging to a cell, like proteases or nucleases. A well-characterised example of this is the ribonuclease inhibitor, which binds to ribonucleases in one of the tightest known protein–protein interactions.[2] Natural enzyme inhibitors can also be poisons and are used as defenses against predators or as ways of killing prey.
Reversible inhibitors
Types of reversible inhibitors
Reversible inhibitors attach to enzymes with non-covalent interactions such as hydrogen bonds, hydrophobic interactions and ionic bonds. Multiple weak bonds between the inhibitor and the active site combine to produce strong and specific binding. In contrast to substrates and irreversible inhibitors, reversible inhibitors generally do not undergo chemical reactions when bound to the enzyme and can be easily removed by dilution or dialysis.
There are four kinds of reversible enzyme inhibitors. They are classified according to the effect of varying the concentration of the enzyme's substrate on the inhibitor.[3][4][1]
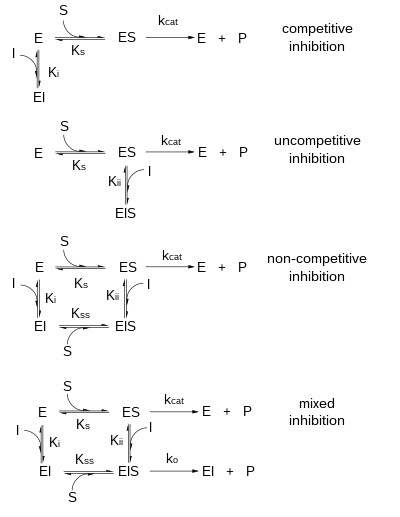
- In competitive inhibition, the substrate and inhibitor cannot bind to the enzyme at the same time, as shown in the figure on the right. This usually results from the inhibitor having an affinity for the active site of an enzyme where the substrate also binds; the substrate and inhibitor compete for access to the enzyme's active site. This type of inhibition can be overcome by sufficiently high concentrations of substrate (Vmax remains constant), i.e., by out-competing the inhibitor. However, the apparent Km will increase as it takes a higher concentration of the substrate to reach the Km point, or half the Vmax. Competitive inhibitors are often similar in structure to the real substrate (see examples below).
- In uncompetitive inhibition, the inhibitor binds only to the substrate-enzyme complex. This type of inhibition causes Vmax to decrease (maximum velocity decreases as a result of removing activated complex) and Km to decrease (due to better binding efficiency as a result of Le Chatelier's principle and the effective elimination of the ES complex thus decreasing the Km which indicates a higher binding affinity).
- In non-competitive inhibition, the binding of the inhibitor to the enzyme reduces its activity but does not affect the binding of substrate. As a result, the extent of inhibition depends only on the concentration of the inhibitor. Vmax will decrease due to the inability for the reaction to proceed as efficiently, but Km will remain the same as the actual binding of the substrate, by definition, will still function properly.
- In mixed inhibition, the inhibitor can bind to the enzyme at the same time as the enzyme's substrate. However, the binding of the inhibitor affects the binding of the substrate, and vice versa. This type of inhibition can be reduced, but not overcome by increasing concentrations of substrate. Although it is possible for mixed-type inhibitors to bind in the active site, this type of inhibition generally results from an allosteric effect where the inhibitor binds to a different site on an enzyme. Inhibitor binding to this allosteric site changes the conformation (i.e., tertiary structure or three-dimensional shape) of the enzyme so that the affinity of the substrate for the active site is reduced.
These types can also be distinguished by the effect of increasing the substrate concentration [S] on the degree of inhibition caused by a given amount of inhibitor. For competitive inhibition the degree of inhibition is reduced by increasing [S], for noncompetitive inhibition the degree of inhibition is unchanged, and for uncompetitive (also called anticompetitive) inhibition the degree of inhibition increases with [S].[6]
Quantitative description of reversible inhibition
Reversible inhibition can be described quantitatively in terms of the inhibitor's binding to the enzyme and to the enzyme-substrate complex, and its effects on the kinetic constants of the enzyme. In the classic Michaelis-Menten scheme below, an enzyme (E) binds to its substrate (S) to form the enzyme–substrate complex ES. Upon catalysis, this complex breaks down to release product P and free enzyme. The inhibitor (I) can bind to either E or ES with the dissociation constants Ki or Ki', respectively.
|
 Kinetic scheme for reversible enzyme inhibitors |
When an enzyme has multiple substrates, inhibitors can show different types of inhibition depending on which substrate is considered. This results from the active site containing two different binding sites within the active site, one for each substrate. For example, an inhibitor might compete with substrate A for the first binding site, but be a non-competitive inhibitor with respect to substrate B in the second binding site.[7]
Measuring the dissociation constants of a reversible inhibitor
As noted above, an enzyme inhibitor is characterised by its two dissociation constants, Ki and Ki', to the enzyme and to the enzyme-substrate complex, respectively. The enzyme-inhibitor constant Ki can be measured directly by various methods; one extremely accurate method is isothermal titration calorimetry, in which the inhibitor is titrated into a solution of enzyme and the heat released or absorbed is measured.[8] However, the other dissociation constant Ki' is difficult to measure directly, since the enzyme-substrate complex is short-lived and undergoing a chemical reaction to form the product. Hence, Ki' is usually measured indirectly, by observing the enzyme activity under various substrate and inhibitor concentrations, and fitting the data[9] to a modified Michaelis–Menten equation
where the modifying factors α and α' are defined by the inhibitor concentration and its two dissociation constants
Thus, in the presence of the inhibitor, the enzyme's effective Km and Vmax become (α/α')Km and (1/α')Vmax, respectively. However, the modified Michaelis-Menten equation assumes that binding of the inhibitor to the enzyme has reached equilibrium, which may be a very slow process for inhibitors with sub-nanomolar dissociation constants. In these cases, it is usually more practical to treat the tight-binding inhibitor as an irreversible inhibitor (see below); however, it can still be possible to estimate Ki' kinetically if Ki is measured independently.
The effects of different types of reversible enzyme inhibitors on enzymatic activity can be visualized using graphical representations of the Michaelis–Menten equation, such as Lineweaver–Burk plots, Eadie-Hofstee plots or Hanes-Woolf plots. For example, in the Lineweaver–Burk plots at the right, the competitive inhibition lines intersect on the y-axis, illustrating that such inhibitors do not affect Vmax. Similarly, the non-competitive inhibition lines intersect on the x-axis, showing these inhibitors do not affect Km. However, it can be difficult to estimate Ki and Ki' accurately from such plots,[10] so it is advisable to estimate these constants using more reliable nonlinear regression methods, as described above.
Reversible inhibitors
Traditionally reversible enzyme inhibitors have been classified as competitive, uncompetitive, or non-competitive, according to their effects on Km and Vmax. These different effects result from the inhibitor binding to the enzyme E, to the enzyme–substrate complex ES, or to both, respectively. The division of these classes arises from a problem in their derivation and results in the need to use two different binding constants for one binding event. The binding of an inhibitor and its effect on the enzymatic activity are two distinctly different things, another problem the traditional equations fail to acknowledge. In noncompetitive inhibition the binding of the inhibitor results in 100% inhibition of the enzyme only, and fails to consider the possibility of anything in between.[11] The common form of the inhibitory term also obscures the relationship between the inhibitor binding to the enzyme and its relationship to any other binding term be it the Michaelis–Menten equation or a dose response curve associated with ligand receptor binding. To demonstrate the relationship the following rearrangement can be made:
This rearrangement demonstrates that similar to the Michaelis–Menten equation, the maximal rate of reaction depends on the proportion of the enzyme population interacting with its substrate.
fraction of the enzyme population bound by substrate
fraction of the enzyme population bound by inhibitor
the effect of the inhibitor is a result of the percent of the enzyme population interacting with inhibitor. The only problem with this equation in its present form is that it assumes absolute inhibition of the enzyme with inhibitor binding, when in fact there can be a wide range of effects anywhere from 100% inhibition of substrate turn over to just >0%. To account for this the equation can be easily modified to allow for different degrees of inhibition by including a delta Vmax term.
or
This term can then define the residual enzymatic activity present when the inhibitor is interacting with individual enzymes in the population. However the inclusion of this term has the added value of allowing for the possibility of activation if the secondary Vmax term turns out to be higher than the initial term. To account for the possibly of activation as well the notation can then be rewritten replacing the inhibitor "I" with a modifier term denoted here as "X".
While this terminology results in a simplified way of dealing with kinetic effects relating to the maximum velocity of the Michaelis–Menten equation, it highlights potential problems with the term used to describe effects relating to the Km. The Km relating to the affinity of the enzyme for the substrate should in most cases relate to potential changes in the binding site of the enzyme which would directly result from enzyme inhibitor interactions. As such a term similar to the one proposed above to modulate Vmax should be appropriate in most situations:[12]
Special cases
- The mechanism of partially competitive inhibition is similar to that of non-competitive, except that the EIS complex has catalytic activity, which may be lower or even higher (partially competitive activation) than that of the enzyme–substrate (ES) complex. This inhibition typically displays a lower Vmax, but an unaffected Km value.[13]
- Uncompetitive inhibition occurs when the inhibitor binds only to the enzyme–substrate complex, not to the free enzyme; the EIS complex is catalytically inactive. This mode of inhibition is rare and causes a decrease in both Vmax and the Km value.[13]
- Substrate and product inhibition is where either the substrate or product of an enzyme reaction inhibit the enzyme's activity. This inhibition may follow the competitive, uncompetitive or mixed patterns. In substrate inhibition there is a progressive decrease in activity at high substrate concentrations. This may indicate the existence of two substrate-binding sites in the enzyme.[1] At low substrate, the high-affinity site is occupied and normal kinetics are followed. However, at higher concentrations, the second inhibitory site becomes occupied, inhibiting the enzyme.[14] Product inhibition is often a regulatory feature in metabolism and can be a form of negative feedback.
- Slow-tight inhibition occurs when the initial enzyme–inhibitor complex EI undergoes isomerisation to a second more tightly held complex, EI*, but the overall inhibition process is reversible. This manifests itself as slowly increasing enzyme inhibition. Under these conditions, traditional Michaelis–Menten kinetics give a false value for Ki, which is time–dependent.[1] The true value of Ki can be obtained through more complex analysis of the on (kon) and off (koff) rate constants for inhibitor association. See irreversible inhibition below for more information.
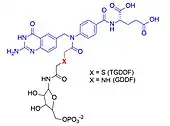
- Bi-substrate analog inhibitors are high affinity and selectivity inhibitors that can be prepared for enzymes that catalyze bi-molecular reactions by capturing the binding energy of each substrate into one molecule.[15][16] For example, in the formyl transfer reactions of purine biosynthesis, a potent multi-substrate adduct inhibitor (MAI) to GAR TFase was prepared synthetically by linking analogs of the glycinamide ribonucleotide (GAR) substrate and the N-10-formyl tetrahydrofolate cofactor together to produce thioglycinamide ribonucleotide dideazafolate (TGDDF),[17] or enzymatically from the natural GAR substrate to yield GDDF.[18] Here the subnanomolar dissociation constant (KD) of TGDDF was greater than predicted presumably due to entropic advantages gained and/or positive interactions acquired through the atoms linking the components. MAIs have also been observed to be produced in cells by reactions of pro-drugs such as isoniazid[19] or enzyme inhibitor ligands (e.g., PTC124) [20] with cellular cofactors such as NADH and ATP respectively.
Examples of reversible inhibitors
As enzymes have evolved to bind their substrates tightly, and most reversible inhibitors bind in the active site of enzymes, it is unsurprising that some of these inhibitors are strikingly similar in structure to the substrates of their targets. Inhibitors of DHFR are prominent examples. Other example of these substrate mimics are the protease inhibitors, a very successful class of antiretroviral drugs used to treat HIV.[21] The structure of ritonavir, a protease inhibitor based on a peptide and containing three peptide bonds, is shown on the right. As this drug resembles the protein that is the substrate of the HIV protease, it competes with this substrate in the enzyme's active site.
Enzyme inhibitors are often designed to mimic the transition state or intermediate of an enzyme-catalyzed reaction. This ensures that the inhibitor exploits the transition state stabilising effect of the enzyme, resulting in a better binding affinity (lower Ki) than substrate-based designs. An example of such a transition state inhibitor is the antiviral drug oseltamivir; this drug mimics the planar nature of the ring oxonium ion in the reaction of the viral enzyme neuraminidase.[22]
However, not all inhibitors are based on the structures of substrates. For example, the structure of another HIV protease inhibitor tipranavir is shown on the left. This molecule is not based on a peptide and has no obvious structural similarity to a protein substrate. These non-peptide inhibitors can be more stable than inhibitors containing peptide bonds, because they will not be substrates for peptidases and are less likely to be degraded.[23]
In drug design it is important to consider the concentrations of substrates to which the target enzymes are exposed. For example, some protein kinase inhibitors have chemical structures that are similar to adenosine triphosphate, one of the substrates of these enzymes. However, drugs that are simple competitive inhibitors will have to compete with the high concentrations of ATP in the cell. Protein kinases can also be inhibited by competition at the binding sites where the kinases interact with their substrate proteins, and most proteins are present inside cells at concentrations much lower than the concentration of ATP. As a consequence, if two protein kinase inhibitors both bind in the active site with similar affinity, but only one has to compete with ATP, then the competitive inhibitor at the protein-binding site will inhibit the enzyme more effectively.[24]
Irreversible inhibitors
Types of irreversible inhibition (covalent inactivation)
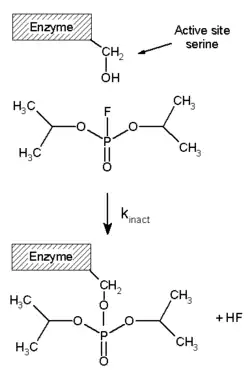
Irreversible inhibitors usually covalently modify an enzyme, and inhibition can therefore not be reversed. Irreversible inhibitors often contain reactive functional groups such as nitrogen mustards, aldehydes, haloalkanes, alkenes, Michael acceptors, phenyl sulfonates, or fluorophosphonates. These nucleophilic groups react with amino acid side chains to form covalent adducts. The residues modified are those with side chains containing nucleophiles such as hydroxyl or sulfhydryl groups; these include the amino acids serine (as in DFP, right), cysteine, threonine, or tyrosine.[25]
Irreversible inhibition is different from irreversible enzyme inactivation. Irreversible inhibitors are generally specific for one class of enzyme and do not inactivate all proteins; they do not function by destroying protein structure but by specifically altering the active site of their target. For example, extremes of pH or temperature usually cause denaturation of all protein structure, but this is a non-specific effect. Similarly, some non-specific chemical treatments destroy protein structure: for example, heating in concentrated hydrochloric acid will hydrolyse the peptide bonds holding proteins together, releasing free amino acids.[26]
Irreversible inhibitors display time-dependent inhibition and their potency therefore cannot be characterised by an IC50 value.[1][27] This is because the amount of active enzyme at a given concentration of irreversible inhibitor will be different depending on how long the inhibitor is pre-incubated with the enzyme. Instead, kobs/[I] values are used,[28] where kobs is the observed pseudo-first order rate of inactivation (obtained by plotting the log of % activity vs. time) and [I] is the concentration of inhibitor. The kobs/[I] parameter is valid as long as the inhibitor does not saturate binding with the enzyme (in which case kobs = kinact).
Analysis of irreversible inhibition
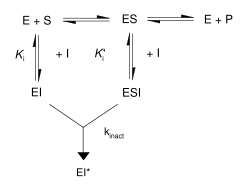
As shown in the figure to the right, irreversible inhibitors have a short instance where they form a reversible non-covalent complex with the enzyme (EI or ESI) and this then reacts to produce the covalently modified "dead-end complex" EI* (an irreversible covalent complex). The rate at which EI* is formed is called the inactivation rate or kinact. Since formation of EI may compete with ES, binding of irreversible inhibitors can be prevented by competition either with substrate or with a second, reversible inhibitor. This protection effect is good evidence of a specific reaction of the irreversible inhibitor with the active site.
The binding and inactivation steps of this reaction are investigated by incubating the enzyme with inhibitor and assaying the amount of activity remaining over time. The activity will be decreased in a time-dependent manner, usually following exponential decay. Fitting these data to a rate equation gives the rate of inactivation at this concentration of inhibitor. This is done at several different concentrations of inhibitor. If a reversible EI complex is involved the inactivation rate will be saturable and fitting this curve will give kinact and Ki.[29]
Another method that is widely used in these analyses is mass spectrometry. Here, accurate measurement of the mass of the unmodified native enzyme and the inactivated enzyme gives the increase in mass caused by reaction with the inhibitor and shows the stoichiometry of the reaction.[30] This is usually done using a MALDI-TOF mass spectrometer. In a complementary technique, peptide mass fingerprinting involves digestion of the native and modified protein with a protease such as trypsin. This will produce a set of peptides that can be analysed using a mass spectrometer. The peptide that changes in mass after reaction with the inhibitor will be the one that contains the site of modification.
Special cases
Not all irreversible inhibitors form covalent adducts with their enzyme targets. Some reversible inhibitors bind so tightly to their target enzyme that they are essentially irreversible. These tight-binding inhibitors may show kinetics similar to covalent irreversible inhibitors. In these cases, some of these inhibitors rapidly bind to the enzyme in a low-affinity EI complex and this then undergoes a slower rearrangement to a very tightly bound EI* complex (see figure above). This kinetic behaviour is called slow-binding.[32] This slow rearrangement after binding often involves a conformational change as the enzyme "clamps down" around the inhibitor molecule. Examples of slow-binding inhibitors include some important drugs, such methotrexate,[33] allopurinol,[34] and the activated form of acyclovir.[35]
Examples of irreversible inhibitors

Diisopropylfluorophosphate (DFP) is shown as an example of an irreversible protease inhibitor in the figure above right. The enzyme hydrolyses the phosphorus–fluorine bond, but the phosphate residue remains bound to the serine in the active site, deactivating it.[36] Similarly, DFP also reacts with the active site of acetylcholine esterase in the synapses of neurons, and consequently is a potent neurotoxin, with a lethal dose of less than 100 mg.[37]
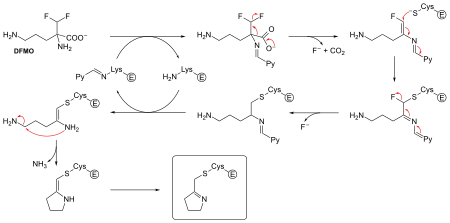
Suicide inhibition is an unusual type of irreversible inhibition where the enzyme converts the inhibitor into a reactive form in its active site. An example is the inhibitor of polyamine biosynthesis, α-difluoromethylornithine or DFMO, which is an analogue of the amino acid ornithine, and is used to treat African trypanosomiasis (sleeping sickness). Ornithine decarboxylase can catalyse the decarboxylation of DFMO instead of ornithine, as shown above. However, this decarboxylation reaction is followed by the elimination of a fluorine atom, which converts this catalytic intermediate into a conjugated imine, a highly electrophilic species. This reactive form of DFMO then reacts with either a cysteine or lysine residue in the active site to irreversibly inactivate the enzyme.[31]
Since irreversible inhibition often involves the initial formation of a non-covalent EI complex, it is sometimes possible for an inhibitor to bind to an enzyme in more than one way. For example, in the figure showing trypanothione reductase from the human protozoan parasite Trypanosoma cruzi, two molecules of an inhibitor called quinacrine mustard are bound in its active site. The top molecule is bound reversibly, but the lower one is bound covalently as it has reacted with an amino acid residue through its nitrogen mustard group.[38]
Discovery and design of inhibitors

New drugs are the products of a long drug development process, the first step of which is often the discovery of a new enzyme inhibitor. In the past the only way to discover these new inhibitors was by trial and error: screening huge libraries of compounds against a target enzyme and hoping that some useful leads would emerge. This brute force approach is still successful and has even been extended by combinatorial chemistry approaches that quickly produce large numbers of novel compounds and high-throughput screening technology to rapidly screen these huge chemical libraries for useful inhibitors.[39]
More recently, an alternative approach has been applied: rational drug design uses the three-dimensional structure of an enzyme's active site to predict which molecules might be inhibitors.[40] These predictions are then tested and one of these tested compounds may be a novel inhibitor. This new inhibitor is then used to try to obtain a structure of the enzyme in an inhibitor/enzyme complex to show how the molecule is binding to the active site, allowing changes to be made to the inhibitor to try to optimise binding. This test and improve cycle is then repeated until a sufficiently potent inhibitor is produced.[41] Computer-based methods of predicting the affinity of an inhibitor for an enzyme are also being developed, such as molecular docking[42] and molecular mechanics.
Uses of inhibitors
Enzyme inhibitors are found in nature and are also designed and produced as part of pharmacology and biochemistry. Natural poisons are often enzyme inhibitors that have evolved to defend a plant or animal against predators. These natural toxins include some of the most poisonous compounds known. Artificial inhibitors are often used as drugs, but can also be insecticides such as malathion, herbicides such as glyphosate, or disinfectants such as triclosan. Other artificial enzyme inhibitors block acetylcholinesterase, an enzyme which breaks down acetylcholine, and are used as nerve agents in chemical warfare.
Chemotherapy
 The structure of sildenafil (Viagra) |
 The coenzyme folic acid (left) compared to the anti-cancer drug methotrexate (right) |
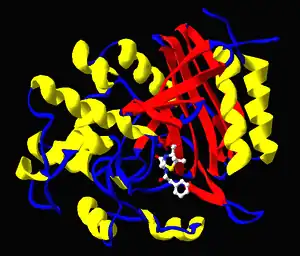 The structure of a complex between penicillin G and the Streptomyces transpeptidase. Generated from PDB 1PWC. |
The most common uses for enzyme inhibitors are as drugs to treat disease. Many of these inhibitors target a human enzyme and aim to correct a pathological condition. However, not all drugs are enzyme inhibitors. Some, such as anti-epileptic drugs, alter enzyme activity by causing more or less of the enzyme to be produced. These effects are called enzyme induction and inhibition and are alterations in gene expression, which is unrelated to the type of enzyme inhibition discussed here. Other drugs interact with cellular targets that are not enzymes, such as ion channels or membrane receptors.
An example of a medicinal enzyme inhibitor is sildenafil (Viagra), a common treatment for male erectile dysfunction. This compound is a potent inhibitor of cGMP specific phosphodiesterase type 5, the enzyme that degrades the signalling molecule cyclic guanosine monophosphate.[43] This signalling molecule triggers smooth muscle relaxation and allows blood flow into the corpus cavernosum, which causes an erection. Since the drug decreases the activity of the enzyme that halts the signal, it makes this signal last for a longer period of time.
Another example of the structural similarity of some inhibitors to the substrates of the enzymes they target is seen in the figure comparing the drug methotrexate to folic acid. Folic acid is a substrate of dihydrofolate reductase, an enzyme involved in making nucleotides that is potently inhibited by methotrexate. Methotrexate blocks the action of dihydrofolate reductase and thereby halts the production of nucleotides. This block of nucleotide biosynthesis is more toxic to rapidly growing cells than non-dividing cells, since a rapidly growing cell has to carry out DNA replication, therefore methotrexate is often used in cancer chemotherapy.[44]
Antibiotics
Drugs also are used to inhibit enzymes needed for the survival of pathogens. For example, bacteria are surrounded by a thick cell wall made of a net-like polymer called peptidoglycan. Many antibiotics such as penicillin and vancomycin inhibit the enzymes that produce and then cross-link the strands of this polymer together.[45] This causes the cell wall to lose strength and the bacteria to burst. In the figure, a molecule of penicillin (shown in a ball-and-stick form) is shown bound to its target, the transpeptidase from the bacteria Streptomyces R61 (the protein is shown as a ribbon diagram).
Antibiotic drug design is facilitated when an enzyme that is essential to the pathogen's survival is absent or very different in humans. In the example above, humans do not make peptidoglycan, therefore inhibitors of this process are selectively toxic to bacteria. Selective toxicity is also produced in antibiotics by exploiting differences in the structure of the ribosomes in bacteria, or how they make fatty acids.
Metabolic control
Enzyme inhibitors are also important in metabolic control. Many metabolic pathways in the cell are inhibited by metabolites that control enzyme activity through allosteric regulation or substrate inhibition. A good example is the allosteric regulation of the glycolytic pathway. This catabolic pathway consumes glucose and produces ATP, NADH and pyruvate. A key step for the regulation of glycolysis is an early reaction in the pathway catalysed by phosphofructokinase-1 (PFK1). When ATP levels rise, ATP binds an allosteric site in PFK1 to decrease the rate of the enzyme reaction; glycolysis is inhibited and ATP production falls. This negative feedback control helps maintain a steady concentration of ATP in the cell. However, metabolic pathways are not just regulated through inhibition since enzyme activation is equally important. With respect to PFK1, fructose 2,6-bisphosphate and ADP are examples of metabolites that are allosteric activators.[46]
Physiological enzyme inhibition can also be produced by specific protein inhibitors. This mechanism occurs in the pancreas, which synthesises many digestive precursor enzymes known as zymogens. Many of these are activated by the trypsin protease, so it is important to inhibit the activity of trypsin in the pancreas to prevent the organ from digesting itself. One way in which the activity of trypsin is controlled is the production of a specific and potent trypsin inhibitor protein in the pancreas. This inhibitor binds tightly to trypsin, preventing the trypsin activity that would otherwise be detrimental to the organ.[47] Although the trypsin inhibitor is a protein, it avoids being hydrolysed as a substrate by the protease by excluding water from trypsin's active site and destabilising the transition state.[48] Other examples of physiological enzyme inhibitor proteins include the barstar inhibitor of the bacterial ribonuclease barnase.[49]
Pesticides
Many pesticides are enzyme inhibitors. Acetylcholinesterase (AChE) is an enzyme found in animals, from insects to humans. It is essential to nerve cell function through its mechanism of breaking down the neurotransmitter acetylcholine into its constituents, acetate and choline. This is somewhat unusual among neurotransmitters as most, including serotonin, dopamine, and norepinephrine, are absorbed from the synaptic cleft rather than cleaved. A large number of AChE inhibitors are used in both medicine and agriculture. Reversible competitive inhibitors, such as edrophonium, physostigmine, and neostigmine, are used in the treatment of myasthenia gravis and in anaesthesia. The carbamate pesticides are also examples of reversible AChE inhibitors. The organophosphate pesticides such as malathion, parathion, and chlorpyrifos irreversibly inhibit acetylcholinesterase.
The herbicide glyphosate is an inhibitor of 3-phosphoshikimate 1-carboxyvinyltransferase,[50] other herbicides, such as the sulfonylureas inhibit the enzyme acetolactate synthase. Both these enzymes are needed for plants to make branched-chain amino acids. Many other enzymes are inhibited by herbicides, including enzymes needed for the biosynthesis of lipids and carotenoids and the processes of photosynthesis and oxidative phosphorylation.[51]
Natural poisons

Animals and plants have evolved to synthesise a vast array of poisonous products including secondary metabolites, peptides and proteins that can act as inhibitors. Natural toxins are usually small organic molecules and are so diverse that there are probably natural inhibitors for most metabolic processes.[52] The metabolic processes targeted by natural poisons encompass more than enzymes in metabolic pathways and can also include the inhibition of receptor, channel and structural protein functions in a cell. For example, paclitaxel (taxol), an organic molecule found in the Pacific yew tree, binds tightly to tubulin dimers and inhibits their assembly into microtubules in the cytoskeleton.[53]
Many natural poisons act as neurotoxins that can cause paralysis leading to death and have functions for defence against predators or in hunting and capturing prey. Some of these natural inhibitors, despite their toxic attributes, are valuable for therapeutic uses at lower doses.[54] An example of a neurotoxin are the glycoalkaloids, from the plant species in the family Solanaceae (includes potato, tomato and eggplant), that are acetylcholinesterase inhibitors. Inhibition of this enzyme causes an uncontrolled increase in the acetylcholine neurotransmitter, muscular paralysis and then death. Neurotoxicity can also result from the inhibition of receptors; for example, atropine from deadly nightshade (Atropa belladonna) that functions as a competitive antagonist of the muscarinic acetylcholine receptors.[55]
Although many natural toxins are secondary metabolites, these poisons also include peptides and proteins. An example of a toxic peptide is alpha-amanitin, which is found in relatives of the death cap mushroom. This is a potent enzyme inhibitor, in this case preventing the RNA polymerase II enzyme from transcribing DNA.[56] The algal toxin microcystin is also a peptide and is an inhibitor of protein phosphatases.[57] This toxin can contaminate water supplies after algal blooms and is a known carcinogen that can also cause acute liver hemorrhage and death at higher doses.[58]
Proteins can also be natural poisons or antinutrients, such as the trypsin inhibitors (discussed above) that are found in some legumes, as shown in the figure above. A less common class of toxins are toxic enzymes: these act as irreversible inhibitors of their target enzymes and work by chemically modifying their substrate enzymes. An example is ricin, an extremely potent protein toxin found in castor oil beans. This enzyme is a glycosidase that inactivates ribosomes. Since ricin is a catalytic irreversible inhibitor, this allows just a single molecule of ricin to kill a cell.[59]
See also
- Activity-based proteomics – a branch of proteomics that uses covalent enzyme inhibitors as reporters to monitor enzyme activity.
- Antimetabolite
- Pharmacophore
- Transition state analog
References
- 1 2 3 4 5 Srinivasan, Bharath (2021). "Explicit Treatment of Non‐Michaelis‐Menten and Atypical Kinetics in Early Drug Discovery*". ChemMedChem. 16 (6): 899–918. doi:10.1002/cmdc.202000791. PMID 33231926. S2CID 227157473.
- ↑ Shapiro R, Vallee BL (February 1991). "Interaction of human placental ribonuclease with placental ribonuclease inhibitor". Biochemistry. 30 (8): 2246–55. doi:10.1021/bi00222a030. PMID 1998683.
- ↑ Berg J., Tymoczko J. and Stryer L. (2002) Biochemistry. W. H. Freeman and Company, ISBN 0-7167-4955-6.
- ↑ Srinivasan, Bharath (2020-09-27). "Words of advice: teaching enzyme kinetics". The FEBS Journal. 288 (7): 2068–2083. doi:10.1111/febs.15537. ISSN 1742-464X. PMID 32981225.
- ↑ Cleland WW (February 1963). "The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory". Biochimica et Biophysica Acta (BBA) - Specialized Section on Enzymological Subjects. 67: 173–87. doi:10.1016/0926-6569(63)90226-8. PMID 14021668.
- ↑ Laidler, Keith J. (1978). Physical Chemistry with Biological Applications. Benjamin/Cummings. p. 437. ISBN 0-8053-5680-0.
- ↑
- Irwin H. Segel, Enzyme Kinetics : Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems. Wiley–Interscience; New edition (1993), ISBN 0-471-30309-7
- ↑ Holdgate GA (July 2001). "Making cool drugs hot: isothermal titration calorimetry as a tool to study binding energetics". BioTechniques. 31 (1): 164–6, 168, 170 passim. PMID 11464510.
- ↑ Leatherbarrow RJ (December 1990). "Using linear and non-linear regression to fit biochemical data". Trends in Biochemical Sciences. 15 (12): 455–8. doi:10.1016/0968-0004(90)90295-M. PMID 2077683.
- ↑ Tseng SJ, Hsu JP (August 1990). "A comparison of the parameter estimating procedures for the Michaelis-Menten model". Journal of Theoretical Biology. 145 (4): 457–64. Bibcode:1990JThBi.145..457T. doi:10.1016/S0022-5193(05)80481-3. PMID 2246896.
- ↑ Walsh R, Martin E, Darvesh S (December 2011). "Limitations of conventional inhibitor classifications". Integrative Biology. 3 (12): 1197–201. doi:10.1039/c1ib00053e. PMID 22038120.
- ↑ Walsh R, Martin E, Darvesh S (May 2007). "A versatile equation to describe reversible enzyme inhibition and activation kinetics: modeling beta-galactosidase and butyrylcholinesterase". Biochimica et Biophysica Acta (BBA) - General Subjects. 1770 (5): 733–46. doi:10.1016/j.bbagen.2007.01.001. PMID 17307293.
- 1 2 Segel, Irwin H. (1993) Enzyme Kinetics : Behavior and Analysis of Rapid Equilibrium and Steady-State Enzyme Systems. Wiley-Interscience; New edition , ISBN 0-471-30309-7.
- ↑ Dixon, M. Webb, E.C., Thorne, C.J.R. and Tipton K.F., Enzymes (3rd edition) Longman, London (1979) p. 126
- ↑ Radzicka A, Wolfenden R (1995). "Transition state and multisubstrate analog inhibitors". Methods in Enzymology. 249: 284–312. doi:10.1016/0076-6879(95)49039-6. PMID 7791615.
- ↑ Schiffer CF, Burke JF, Besarab A, Lasker N, Simenhoff ML (January 1977). "Amylase/creatinine clearance fraction in patients on chronic hemodialysis". Annals of Internal Medicine. 86 (1): 65–6. doi:10.7326/0003-4819-86-1-65. PMID 319722.
- ↑ Inglese J, Blatchly RA, Benkovic SJ (May 1989). "A multisubstrate adduct inhibitor of a purine biosynthetic enzyme with a picomolar dissociation constant". Journal of Medicinal Chemistry. 32 (5): 937–40. doi:10.1021/jm00125a002. PMID 2709379.
- ↑ Inglese J, Benkovic SJ (1991). "Multisubstrate Adduct Inhibitors of Glycinamide Ribonucleotide Transformylase: Synthetic and Enzyme Generated". Tetrahedron. 47 (14–15): 2351–2364. doi:10.1016/S0040-4020(01)81773-7.
- ↑ Rozwarski DA, Grant GA, Barton DH, Jacobs WR, Sacchettini JC (January 1998). "Modification of the NADH of the isoniazid target (InhA) from Mycobacterium tuberculosis". Science. 279 (5347): 98–102. Bibcode:1998Sci...279...98R. doi:10.1126/science.279.5347.98. PMID 9417034.
- ↑ Auld DS, Lovell S, Thorne N, Lea WA, Maloney DJ, Shen M, Rai G, Battaile KP, Thomas CJ, Simeonov A, Hanzlik RP, Inglese J (March 2010). "Molecular basis for the high-affinity binding and stabilization of firefly luciferase by PTC124". Proceedings of the National Academy of Sciences of the United States of America. 107 (11): 4878–83. Bibcode:2010PNAS..107.4878A. doi:10.1073/pnas.0909141107. PMC 2841876. PMID 20194791.
- ↑ Hsu JT, Wang HC, Chen GW, Shih SR (2006). "Antiviral drug discovery targeting to viral proteases". Current Pharmaceutical Design. 12 (11): 1301–14. doi:10.2174/138161206776361110. PMID 16611117.
- ↑ Lew W, Chen X, Kim CU (June 2000). "Discovery and development of GS 4104 (oseltamivir): an orally active influenza neuraminidase inhibitor". Current Medicinal Chemistry. 7 (6): 663–72. doi:10.2174/0929867003374886. PMID 10702632.
- ↑ Fischer PM (October 2003). "The design, synthesis and application of stereochemical and directional peptide isomers: a critical review". Current Protein & Peptide Science. 4 (5): 339–56. doi:10.2174/1389203033487054. PMID 14529528.
- ↑ Bogoyevitch MA, Barr RK, Ketterman AJ (December 2005). "Peptide inhibitors of protein kinases-discovery, characterisation and use". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 1754 (1–2): 79–99. doi:10.1016/j.bbapap.2005.07.025. PMID 16182621.
- ↑ Lundblad RL (2004). Chemical reagents for protein modification (3rd ed.). CRC Press. ISBN 978-0-8493-1983-9.
- ↑ Price N, Hames B, Rickwood D (1996). Proteins LabFax. BIOS Scientific Publishers. ISBN 978-0-12-564710-6.
- ↑ Srinivasan, Bharath; Kantae, Vasudev; Robinson, James (2020-04-13). "Resurrecting the phoenix: When an assay fails". Medicinal Research Reviews. 40 (5): 1776–1793. doi:10.1002/med.21670. ISSN 0198-6325. PMID 32285494. S2CID 215758654.
- ↑ Adam GC, Cravatt BF, Sorensen EJ (January 2001). "Profiling the specific reactivity of the proteome with non-directed activity-based probes". Chemistry & Biology. 8 (1): 81–95. doi:10.1016/S1074-5521(00)90060-7. PMID 11182321.
- ↑ Maurer T, Fung HL (2000). "Comparison of methods for analyzing kinetic data from mechanism-based enzyme inactivation: application to nitric oxide synthase". AAPS PharmSci. 2 (1): 68–77. doi:10.1208/ps020108. PMC 2751003. PMID 11741224.
- ↑ Loo JA, DeJohn DE, Du P, Stevenson TI, Ogorzalek Loo RR (July 1999). "Application of mass spectrometry for target identification and characterization". Medicinal Research Reviews. 19 (4): 307–19. doi:10.1002/(SICI)1098-1128(199907)19:4<307::AID-MED4>3.0.CO;2-2. PMID 10398927.
- 1 2 Poulin R, Lu L, Ackermann B, Bey P, Pegg AE (January 1992). "Mechanism of the irreversible inactivation of mouse ornithine decarboxylase by alpha-difluoromethylornithine. Characterization of sequences at the inhibitor and coenzyme binding sites". The Journal of Biological Chemistry. 267 (1): 150–8. doi:10.1016/S0021-9258(18)48472-4. PMID 1730582.
- ↑ Szedlacsek SE, Duggleby RG (1995). "[6] Kinetics of slow and tight-binding inhibitors". Kinetics of slow and tight-binding inhibitors. Methods in Enzymology. Vol. 249. pp. 144–80. doi:10.1016/0076-6879(95)49034-5. ISBN 978-0-12-182150-0. PMID 7791610.
- ↑ Stone SR, Morrison JF (February 1986). "Mechanism of inhibition of dihydrofolate reductases from bacterial and vertebrate sources by various classes of folate analogues". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 869 (3): 275–85. doi:10.1016/0167-4838(86)90067-1. PMID 3511964.
- ↑ Pick FM, McGartoll MA, Bray RC (January 1971). "Reaction of formaldehyde and of methanol with xanthine oxidase". European Journal of Biochemistry. 18 (1): 65–72. doi:10.1111/j.1432-1033.1971.tb01215.x. PMID 4322209.
- ↑ Reardon JE (November 1989). "Herpes simplex virus type 1 and human DNA polymerase interactions with 2'-deoxyguanosine 5'-triphosphate analogues. Kinetics of incorporation into DNA and induction of inhibition". The Journal of Biological Chemistry. 264 (32): 19039–44. doi:10.1016/S0021-9258(19)47263-3. PMID 2553730.
- ↑ Cohen JA, Oosterbaan RA, Berends F (1967). "[81] Organophosphorus compounds". Enzyme Structure. Methods in Enzymology. Vol. 11. pp. 686–702. doi:10.1016/S0076-6879(67)11085-9. ISBN 978-0-12-181860-9. Archived from the original on 2018-02-28.
- ↑ Brenner GM (2000). Pharmacology (1st ed.). Philadelphia, PA: W.B. Saunders. ISBN 978-0-7216-7757-6.
- ↑ Saravanamuthu A, Vickers TJ, Bond CS, Peterson MR, Hunter WN, Fairlamb AH (July 2004). "Two interacting binding sites for quinacrine derivatives in the active site of trypanothione reductase: a template for drug design". The Journal of Biological Chemistry. 279 (28): 29493–500. doi:10.1074/jbc.M403187200. PMC 3491871. PMID 15102853.
- ↑ Koppitz M, Eis K (June 2006). "Automated medicinal chemistry". Drug Discovery Today. 11 (11–12): 561–8. doi:10.1016/j.drudis.2006.04.005. PMID 16713909.
- ↑ Scapin G (2006). "Structural biology and drug discovery". Current Pharmaceutical Design. 12 (17): 2087–97. doi:10.2174/138161206777585201. PMID 16796557.
- ↑ Gohlke H, Klebe G (August 2002). "Approaches to the description and prediction of the binding affinity of small-molecule ligands to macromolecular receptors". Angewandte Chemie. 41 (15): 2644–76. doi:10.1002/1521-3773(20020802)41:15<2644::AID-ANIE2644>3.0.CO;2-O. PMID 12203463.
- ↑ Glen RC, Allen SC (May 2003). "Ligand-protein docking: cancer research at the interface between biology and chemistry". Current Medicinal Chemistry. 10 (9): 763–7. doi:10.2174/0929867033457809. PMID 12678780.
- ↑ Maggi M, Filippi S, Ledda F, Magini A, Forti G (August 2000). "Erectile dysfunction: from biochemical pharmacology to advances in medical therapy". European Journal of Endocrinology. 143 (2): 143–54. doi:10.1530/eje.0.1430143. PMID 10913932.
- ↑ McGuire JJ (2003). "Anticancer antifolates: current status and future directions". Current Pharmaceutical Design. 9 (31): 2593–613. doi:10.2174/1381612033453712. PMID 14529544.
- ↑ Katz AH, Caufield CE (2003). "Structure-based design approaches to cell wall biosynthesis inhibitors". Current Pharmaceutical Design. 9 (11): 857–66. doi:10.2174/1381612033455305. PMID 12678870.
- ↑ Okar DA, Lange AJ (1999). "Fructose-2,6-bisphosphate and control of carbohydrate metabolism in eukaryotes". BioFactors. 10 (1): 1–14. doi:10.1002/biof.5520100101. PMID 10475585. S2CID 24586866.
- ↑ Price NC, Stevens L (1999). Fundamentals of enzymology : the cell and molecular biology of catalytic proteins (3rd ed.). Oxford University Press. ISBN 978-0-19-850229-6.
- ↑ Smyth TP (August 2004). "Substrate variants versus transition state analogues as noncovalent reversible enzyme inhibitors". Bioorganic & Medicinal Chemistry. 12 (15): 4081–8. doi:10.1016/j.bmc.2004.05.041. PMID 15246086.
- ↑ Hartley RW (November 1989). "Barnase and barstar: two small proteins to fold and fit together". Trends in Biochemical Sciences. 14 (11): 450–4. doi:10.1016/0968-0004(89)90104-7. PMID 2696173.
- ↑ Tan S, Evans R, Singh B (March 2006). "Herbicidal inhibitors of amino acid biosynthesis and herbicide-tolerant crops". Amino Acids. 30 (2): 195–204. doi:10.1007/s00726-005-0254-1. PMID 16547651. S2CID 2358278.
- ↑ Duke SO (July 1990). "Overview of herbicide mechanisms of action". Environmental Health Perspectives. 87: 263–71. doi:10.2307/3431034. JSTOR 3431034. PMC 1567841. PMID 1980104.
- ↑ Tan G, Gyllenhaal C, Soejarto DD (March 2006). "Biodiversity as a source of anticancer drugs". Current Drug Targets. 7 (3): 265–77. doi:10.2174/138945006776054942. PMID 16515527.
- ↑ Abal M, Andreu JM, Barasoain I (June 2003). "Taxanes: microtubule and centrosome targets, and cell cycle dependent mechanisms of action". Current Cancer Drug Targets. 3 (3): 193–203. doi:10.2174/1568009033481967. PMID 12769688.
- ↑ Hostettmann K, Borloz A, Urbain A, Marston A (2006). "Natural Product Inhibitors of Acetylcholinesterase". Current Organic Chemistry. 10 (8): 825–847. doi:10.2174/138527206776894410.
- ↑ DeFrates LJ, Hoehns JD, Sakornbut EL, Glascock DG, Tew AR (January 2005). "Antimuscarinic intoxication resulting from the ingestion of moonflower seeds". The Annals of Pharmacotherapy. 39 (1): 173–6. doi:10.1345/aph.1D536. PMID 15572604. S2CID 36465515.
- ↑ Vetter J (January 1998). "Toxins of Amanita phalloides". Toxicon. 36 (1): 13–24. doi:10.1016/S0041-0101(97)00074-3. PMID 9604278.
- ↑ Holmes CF, Maynes JT, Perreault KR, Dawson JF, James MN (November 2002). "Molecular enzymology underlying regulation of protein phosphatase-1 by natural toxins". Current Medicinal Chemistry. 9 (22): 1981–9. doi:10.2174/0929867023368827. PMID 12369866.
- ↑ Bischoff K (October 2001). "The toxicology of microcystin-LR: occurrence, toxicokinetics, toxicodynamics, diagnosis and treatment". Veterinary and Human Toxicology. 43 (5): 294–7. PMID 11577938.
- ↑ Hartley MR, Lord JM (September 2004). "Cytotoxic ribosome-inactivating lectins from plants". Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics. 1701 (1–2): 1–14. doi:10.1016/j.bbapap.2004.06.004. PMID 15450171.
External links
- Web tutorial on enzyme inhibition, Tutorial by Dr Peter Birch of the University of Paisley, containing very clear animations
- Symbolism and Terminology in Enzyme Kinetics, Recommendations of the Nomenclature Committee of the International Union of Biochemistry (NC-IUB) on enzyme inhibition terminology
- PubChem from NCBI, Database of drugs and enzyme inhibitors
- BRENDA Archived 2008-12-11 at the Wayback Machine, Database of enzymes giving lists of known inhibitors for each entry
- Enzymes, Kinetics and Diagnostic Use, On-line lecture concentrating on medical applications of enzyme inhibitors: by Dr. Michael W. King of the IU School of Medicine
- BindingDB, a public database of measured protein-ligand binding affinities.
- Enzyme Inhibition Animated Exercise (tutorial + quizzes).