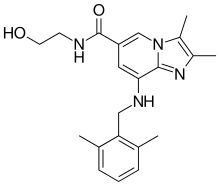
Linaprazan
 | |
| Clinical data | |
|---|---|
| Other names | AZD-0865 |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| Chemical and physical data | |
| Formula | C21H26N4O2 |
| Molar mass | 366.465 g·mol−1 |
Linaprazan is an experimental drug for the treatment of gastroesophageal reflux disease (GERD). Unlike the proton-pump inhibitors (PPIs) which are typically used to treat GERD, linaprazan is a potassium-competitive acid blocker (P-CAB).[1][2] Linaprazan was developed by AstraZeneca, but it was not successful in clinical trials.[3]
The drug was then licensed to Cinclus Pharma,[4] which is now investigating linaprazan glurate, a prodrug of linaprazan which is expected to have a longer biological half-life than linaprazan itself.[4]
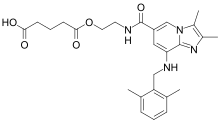 Chemical structure of linaprazan glurate
Chemical structure of linaprazan glurate
References
- ↑ Rawla P, Sunkara T, Ofosu A, Gaduputi V (December 2018). "Potassium-competitive acid blockers - are they the next generation of proton pump inhibitors?". World Journal of Gastrointestinal Pharmacology and Therapeutics. 9 (7): 63–68. doi:10.4292/wjgpt.v9.i7.63. PMC 6305499. PMID 30595950.
- ↑ "Linaprazan". Inxight Drugs. National Center for Advancing Translational Sciences.
- ↑ Tong A (4 March 2020). "Can reformulation of an AstraZeneca castoff rival Takeda's new heartburn drug? Here's a $26M bet on yes". endpts.com.
- 1 2 "Linaprazan glurate". Cinclus Pharma.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.