Revaprazan
 | |
| Clinical data | |
|---|---|
| Trade names | Revanex[1] |
| Other names | YH1885 |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
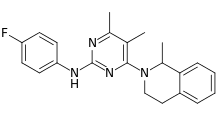
| Formula | C22H23FN4 |
| Molar mass | 362.452 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Revaprazan (trade name Revanex) is a drug that reduces gastric acid secretion which is used for the treatment of gastritis.[2] It acts as an acid pump antagonist[3] (potassium-competitive acid blocker). Revaprazan is approved for use in South Korea,[4] but is not approved in Europe or the United States.
References
- ↑ "Revaprazan". drugs.com.
- ↑ Kim HK, Park SH, Cheung DY, Cho YS, Kim JI, Kim SS, Chae HS, Kim JK, Chung IS (Oct 2010). "Clinical trial: inhibitory effect of revaprazan on gastric acid secretion in healthy male subjects". J Gastroenterol Hepatol. 25 (10): 1618–1625. doi:10.1111/j.1440-1746.2010.06408.x. PMID 20880169. S2CID 41932174.
- ↑ Yu KS, Bae KS, Shon JH, Cho JY, Yi SY, Chung JY, Lim HS, Jang IJ, Shin SG, Song KS, Moon BS (Jan 2004). "Pharmacokinetic and pharmacodynamic evaluation of a novel proton pump inhibitor, YH1885, in healthy volunteers". J Clin Pharmacol. 44 (1): 73–82. doi:10.1177/0091270003261321. PMID 14681344. S2CID 19658496.
- ↑ "Revaprazan Yuhan registered, South Korea (gastritis)". R & D Focus Drug News. September 25, 2006. Archived from the original on April 29, 2014.
This article is issued from Offline. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.