Aceglutamide
 | |
| Names | |
|---|---|
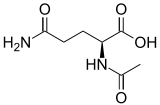
| Preferred IUPAC name
2-Acetamido-5-amino-5-oxopentanoic acid | |
| Other names
2-(Acetylamino)-glutaramidic acid α-N-Acetylglutamine;[1] | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.017.862 |
| EC Number |
|
| KEGG | |
| MeSH | aceglutamide |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C7H12N2O4 |
| Molar mass | 188.183 g·mol−1 |
| Appearance | White crystals |
| Melting point | 197 °C (387 °F; 470 K) |
| Related compounds | |
Related alkanoic acids |
|
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aceglutamide (brand name Neuramina), or aceglutamide aluminum (brand name Glumal), also known as acetylglutamine, is a psychostimulant, nootropic, and antiulcer agent that is marketed in Spain and Japan.[1][2][3][4] It is an acetylated form of the amino acid L-glutamine, the precursor of glutamate in the body and brain.[5] Aceglutamide functions as a prodrug to glutamine with improved potency and stability.[5]
Aceglutamide is used as a psychostimulant and nootropic, while aceglutamide aluminum is used in the treatment of ulcers.[6][7][8][9] Aceglutamide can also be used as a liquid-stable source of glutamine to prevent damage from protein energy malnutrition.[10][11][12] The drug has shown neuroprotective effects in an animal model of cerebral ischemia.[5]
See also
- Aceburic acid
- Aceturic acid
- N-Acetylglutamic acid
References
- 1 2 Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 3–. ISBN 978-1-4757-2085-3.
- ↑ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 6–. ISBN 978-3-88763-075-1.
- ↑ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 35–. ISBN 978-0-8155-1856-3.
- ↑ Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 3–. ISBN 978-94-011-4439-1.
- 1 2 3 Zhang R, Yang N, Ji C, Zheng J, Liang Z, Hou CY, et al. (2015). "Neuroprotective effects of Aceglutamide on motor function in a rat model of cerebral ischemia and reperfusion". Restorative Neurology and Neuroscience. 33 (5): 741–59. doi:10.3233/RNN-150509. PMID 26444640.
- ↑ Ito M, Yokochi E, Kobayashi C, Suzuki Y (April 1982). "[Studies on defensive factors of experimental ulcers (2). Increasing action of aceglutamide aluminum on defensive factors in acetic acid ulcers of rats (author's transl)]". Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica. 79 (4): 327–34. doi:10.1254/fpj.79.327. PMID 7095654.
- ↑ Harada M, Yano S (1974). "Inhibitory effect of N-acetyl-L-glutamine aluminum complex (KW-110) and related compounds on gastric erosion and motility in stressed animals". Oyo Yakuri. 8 (1): 1–6.
- ↑ Varas Lorenzo MJ, López Martínez A, Gordillo Bernal J, Mundet Surroca J (August 1991). "[Comparative study of 3 drugs (aceglutamide aluminum, zinc acexamate, and magaldrate) in the long-term maintenance treatment (1 year) of peptic ulcer]". Revista Espanola de Enfermedades Digestivas. 80 (2): 91–4. PMID 1790087.
- ↑ Tanaka H, Shuto K, Marumo H (April 1982). "Effect of N-acetyl-L-glutamine aluminum complex (KW-110), an antiulcer agent, on the non-steroidal anti-inflammatory drug-induced exacerbation of gastric ulcer in rats". Japanese Journal of Pharmacology. 32 (2): 307–13. doi:10.1254/jjp.32.307. PMID 7098147.
- ↑ López-Pedrosa JM, Manzano M, Baxter JH, Rueda R (March 2007). "N-acetyl-L-glutamine, a liquid-stable source of glutamine, partially prevents changes in body weight and on intestinal immunity induced by protein energy malnutrition in pigs". Digestive Diseases and Sciences. 52 (3): 650–8. doi:10.1007/s10620-006-9500-y. PMID 17253138. S2CID 37484555.
- ↑ JP 10101576
- ↑ US 2003099722