Lingual gyrus
| Lingual gyrus | |
|---|---|
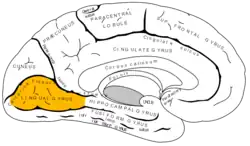 Medial surface of left cerebral hemisphere. (Lingual gyrus visible at left.) | |
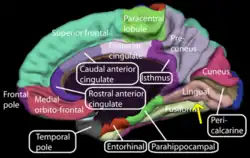 Medial surface of right cerebral hemisphere. (Lingual gyrus visible at right.) | |
| Details | |
| Part of | Occipital lobe |
| Artery | Posterior cerebral |
| Identifiers | |
| Latin | gyrus lingualis |
| NeuroNames | 158 |
| NeuroLex ID | birnlex_740 |
| TA98 | A14.1.09.226 |
| TA2 | 5487 |
| FMA | 61904 |
| Anatomical terms of neuroanatomy | |
The lingual gyrus, also known as the medial occipitotemporal gyrus,[1] is a brain structure that is linked to processing vision, especially related to letters. It is thought to also play a role in analysis of logical conditions (i.e., logical order of events) and encoding visual memories. It is named after its shape, which is somewhat similar to a tongue. Contrary to the name, the region has little to do with speech.
It is believed that a hypermetabolism of the lingual gyrus is associated with visual snow.[2]
Location
The lingual gyrus of the occipital lobe lies between the calcarine sulcus and the posterior part of the collateral sulcus; behind, it reaches the occipital pole; in front, it is continued on to the tentorial surface of the temporal lobe, and joins the parahippocampal gyrus.[3]
Function
Role in vision
This region is believed to play an important role in vision and dreaming. Visual memory dysfunction and visuo-limbic disconnection have been shown in cases where the lingual gyrus has been damaged (due to stroke or other traumatic brain injuries). Further, impaired visual memory is related to either damage to the region or disconnections between the gyrus and other brain structures.[4] Hypermetabolism in the lingual gyrus has been associated with visual snow syndrome.[5]
Lingual gyrus activation has been linked to encoding of complex images. Subjects were scanned using fMRI while looking at pictures. The images were emotionally neutral, with no people in close-up. Subjects were tasked with memorizing the images for recognition at a later date. Data from the fMRI showed activation in several structures, notably the lingual gyrus. Similar activation was recorded during the recollection several weeks later.[6] It has also been shown that activation of the ventral occipitotemporal cortex, including the lingual gyrus, is related to the processing of visual information about parts of human faces.[7] Furthermore, the left lingual gyrus activates during memorizing and maintaining images of human faces in working memory.[8][9]
Activation of the lingual gyrus has been shown in selective visual attention studies. Subjects were tasked with memorizing symbols in certain visual fields while ignoring those in others. In some subjects, the lingual gyrus was activated. The hemispheric activation of the structure was dependent on which visual field the subject was focused on.[10] Hemispheric-dependent gyrus activation has also been shown by isolating visual fields rather than by diverting focus.[11]
Role in word processing
The lingual gyrus is a structure in the visual cortex that plays an important role in the identification and recognition of words.[12] Studies have implicated the lingual gyrus as being involved in modulating visual stimuli (especially letters) but not whether or not the stimulus was a word. Further, the gyrus is related to the naming of stimuli.[13][14][15] Furthermore, the gyrus has shown significant activation when moving from high to low contrast words as well as a correlation between word length and regional activation.[12]
In addition to recognition of letters, the region has been linked to semantic processing. Subjects with aphasia were tested with a variety of aphasia tests while undergoing fMRI to determine which areas were affected. Repetition of stimuli led to modulation in the lingual gyrus in subjects not afflicted, while those with aphasia showed significantly less modulation.[16]
Similarly, the region is activated by non-verbal, logic-based conditions. Subjects tasked with attributing intentions to characters in comic strips showed activation in the gyrus when comparing physical logic with and without characters. For example: if a subject was intended to determine what a character will do, the region will activate. Conversely, if the comic depicted a physical event without characters, the region was relatively dormant.[17]
Additional studies have shown a relationship between memorization and activation in the gyrus. When subjects were tasked with pairing abstract nouns with either visual imagery or sentence generation, many areas in the occipital lobe – namely the lingual gyrus – showed task-selective memory effects. This effect was primarily linked to visual imagery, as there were no significant effects associated with sentence generation.[18] This link between memory and the gyrus extends to retrieval fluency in children, as well. Studies have shown elevated signals in the lingual gyrus when subjects were tasked with retrieval of facts while problem solving. Control samples show the activation is not linked to the problem solving itself, rather the recollection. This suggests a potential link between the lingual gyrus and hippocampal regions in the brain.[19] Furthermore, the gyrus is potentially linked to the amygdala. Gyrus activation was observed when subjects were tasked with verbalizing high-emotion words in contrast to neutral-emotion words.[20] A second study linked the regions with high-emotion images. When subjects were shown emotional images, the amygdala and lingual gyrus both activated significantly more when compared to neutral-emotion images.[21]
Additional images
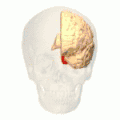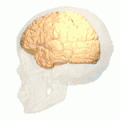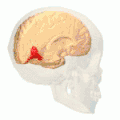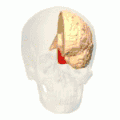 Position of lingual gyrus (shown in red).
Position of lingual gyrus (shown in red).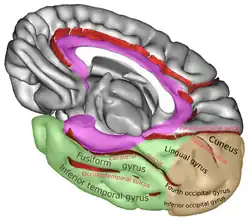 Gyri and sulci of occipital and temporal lobe.
Gyri and sulci of occipital and temporal lobe.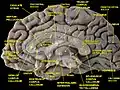 Medial surface of cerebral hemisphere. Medial view. Deep dissection.
Medial surface of cerebral hemisphere. Medial view. Deep dissection. Inner lingual gyrus, shown in the right cerebral hemisphere.
Inner lingual gyrus, shown in the right cerebral hemisphere. Lingual gyrus highlighted in green on sagittal T1 MRI images
Lingual gyrus highlighted in green on sagittal T1 MRI images Lingual gyrus highlighted in green on coronal T1 MRI images
Lingual gyrus highlighted in green on coronal T1 MRI images Lingual gyrus highlighted in green on transversal T1 MRI images
Lingual gyrus highlighted in green on transversal T1 MRI images
See also
References
- ↑ Standring (2015). Gray's Anatomy: The Anatomical Basis of Clinical Practice 41st edition. Elsevier.
- ↑ Gray. Henry. Peter L. Williams. and Henry Gray. Gray's Anatomy. Edinburgh: C. Livingstone. 1989. Print.
- ↑ Mendoza. John E.. and Anne L. Foundas. Clinical Neuroanatomy: A Neurobehavioral Approach. New York: Springer. 2008. Print.
- ↑ Bogousslavsky, J.; Miklossy, J.; Deruaz, J. P.; Assal, G.; Regli, F. (May 1987). "Lingual and fusiform gyri in visual processing: a clinico-pathologic study of superior altitudinal hemianopia". Journal of Neurology, Neurosurgery, and Psychiatry. 50 (5): 607–614. doi:10.1136/jnnp.50.5.607. ISSN 0022-3050. PMC 1031973. PMID 3585386.
- ↑ Schankin, Christoph J.; Maniyar, Farooq H.; Sprenger, Till; Chou, Denise E.; Eller, Michael; Goadsby, Peter J. (June 2014). "The relation between migraine, typical migraine aura and "visual snow"". Headache. 54 (6): 957–966. doi:10.1111/head.12378. ISSN 1526-4610. PMID 24816400.
- ↑ Machielsen, Willem C. M.; Rombouts, Serge A. R. B.; Barkhof, Frederik; Scheltens, Philip; Witter, Menno P. (2000). "fMRI of visual encoding: Reproducibility of activation". Human Brain Mapping. 9 (3): 156–164. doi:10.1002/(SICI)1097-0193(200003)9:3<156::AID-HBM4>3.0.CO;2-Q. ISSN 1097-0193. PMC 6871840. PMID 10739366.
- ↑ McCarthy, G.; Puce, A.; Belger, A.; Allison, T. (1999). "Electrophysiological studies of human face perception. II: Response properties of face-specific potentials generated in occipitotemporal cortex". Cereb Cortex. 9 (5): 431–44. doi:10.1093/cercor/9.5.431. PMID 10450889.
- ↑ Kozlovskiy, S.A.; Pyasik, M.M.; Korotkova, A.V.; Vartanov, A.V.; Glozman, J.M; Kiselnikov, A.A. (2014). "Activation of left lingual gyrus related to working memory for schematic faces". International Journal of Psychophysiology. 94 (2): 241. doi:10.1016/j.ijpsycho.2014.08.928.
- ↑ Kozlovskiy, S.A.; Pyasik, M.M.; Korotkova, A.V.; Vartanov, A.V.; Kiselnikov, A.A.; Glozman, J.M. (2014). "Selective Involvement of Lingual Gyrus in Working Memory and Perception of Different Types of Visual Stimuli". Journal of the International Neuropsychological Society. 20 (S2): 43. doi:10.1017/S1355617714000915.
- ↑ Mangun, G. R., Buonocore, M. H., Girelli, M., & Jha, A. P. (1998). ERP and fMRI measures of visual spatial selective attention. Hum Brain Mapp, 6(5-6), 383-389.
- ↑ Driver, J., & Spence, C. (2000). Multisensory perception: Beyond modularity and convergence. Current Biology, 10(20), R731-R735. doi: 10.1016/s0960-9822(00)00740-5
- 1 2 Mechelli. A.. Humphreys. G. W.. Mayall. K.. Olson. A.. 8. Price. C. J. (2000). Differential effects of word length and visual contrast in the fusiform and lingual gyri during reading. Proc Biol Sci. 267(1455). 1909-1913.
- ↑ Howard. D.. Patterson. K..Wise. R.. Brown.W. D.. Friston. K.. Weiller. C. 8. Frackowiak. R. S. J. 1992 The cortical localization of the lexicons: positron emission tomography evidence. Brain 115. 1769-1782.
- ↑ Price. C. J.. Wise. R.. Watson. J.. Patterson. K.. Howard. D. 8. Frackowiak. R. S. J. 1994 Brain activity during reading: the effects of task and exposure duration. Brain 117. 1255-1269.
- ↑ Bookheimer. S. Y.. Zefro. T. A.. Blaxton. T.. Gaillard. W. 8. Theodore.W. 1995 Regional cerebral blood flow during object naming and word reading. Hum. Brain Mapp. 3. 93"106.
- ↑ Heath, S., McMahon, K. L., Nickels, L., Angwin, A., Macdonald, A. D., van Hees, S., . . . Copland, D. A. (2012). Neural mechanisms underlying the facilitation of naming in aphasia using a semantic task: an fMRI study. BMC Neurosci, 13(1), 98. doi: 10.1186/1471-2202-13-98
- ↑ Brunet, E., Sarfati, Y., Hardy-Baylé, M.-C., & Decety, J. (2000). A PET Investigation of the Attribution of Intentions with a Nonverbal Task. NeuroImage, 11(2), 157-166. doi: 10.1006/nimg.1999.0525
- ↑ Leshikar, E. D., Duarte, A., & Hertzog, C. (2012). Task-Selective Memory Effects for Successfully Implemented Encoding Strategies. [Article]. PLoS ONE, 7(5). doi: 10.1371/journal.pone.0038160
- ↑ Cho, S., Metcalfe, A. W. S., Young, C. B., Ryali, S., Geary, D. C., & Menon, V. (2012). Hippocampal-Prefrontal Engagement and Dynamic Causal Interactions in the Maturation of Children's Fact Retrieval. [Article]. Journal of Cognitive Neuroscience, 24(9), 1849-1866.
- ↑ Isenberg, N., Silbersweig, D., Engelien, A., Emmerich, S., Malavade, K., Beattie, B., . . . Stern, E. (1999). Linguistic threat activates the human amygdala. Proc Natl Acad Sci U S A, 96(18), 10456-10459.
- ↑ Kehoe, E. G., Toomey, J. M., Balsters, J. H., & Bokde, A. L. (2012). Healthy aging is associated with increased neural processing of positive valence but attenuated processing of emotional arousal: an fMRI study. Neurobiol Aging. doi: 10.1016/j.neurobiolaging.2012.07.006
| Wikimedia Commons has media related to Lingual gyrus. |