Orbitofrontal cortex
| Orbitofrontal cortex | |
|---|---|
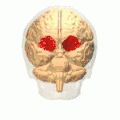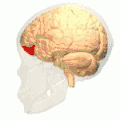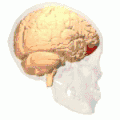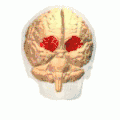
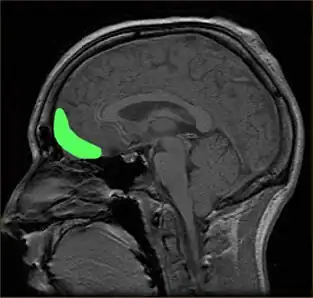 Approximate location of the OFC shown on a sagittal MRI | |
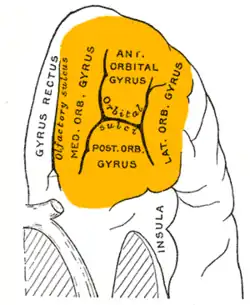 Orbital surface of left frontal lobe. | |
| Details | |
| Part of | Frontal lobe |
| Identifiers | |
| Latin | cortex orbitofrontalis |
| NeuroNames | 91 |
| NeuroLex ID | birnlex_1049 |
| FMA | 242003 |
| Anatomical terms of neuroanatomy | |
The orbitofrontal cortex (OFC) is a prefrontal cortex region in the frontal lobes of the brain which is involved in the cognitive process of decision-making. In non-human primates it consists of the association cortex areas Brodmann area 11, 12 and 13; in humans it consists of Brodmann area 10, 11 and 47.[1]
The OFC is considered anatomically synonymous with the ventromedial prefrontal cortex.[2] Therefore, the region is distinguished due to the distinct neural connections and the distinct functions it performs.[3] It is defined as the part of the prefrontal cortex that receives projections from the medial dorsal nucleus of the thalamus, and is thought to represent emotion, taste, olfaction and reward in decision making.[4][5][6][7][8][9][10][11] It gets its name from its position immediately above the orbits in which the eyes are located. Considerable individual variability has been found in the OFC of humans.[12] A related area is found in rodents.[13]
Structure
The OFC is divided into multiple broad regions distinguished by cytoarchitecture, including brodmann area 47/12, brodmann area 11, brodmann area 14, brodmann area 13, and brodmann area 10.[14] Four gyri are split by a complex of sulci that most frequently resembles a "H" or a "K" pattern. Extending along the rostro-caudal axis, two sulci, the lateral and orbital sulci, are usually connected by the transverse orbital sulcus, which extends along a medial-lateral axis. Most medially, the medial orbital gyrus is separated from the gyrus rectus by the olfactory sulcus.[15] Anteriorly, both the gyrus rectus and the medial part of the medial orbital gyrus consist of area 11(m), and posteriorly, area 14. The posterior orbital gyrus consists mostly of area 13, and is bordered medially and laterally by the anterior limbs of the medial and lateral orbital sulci. Area 11 makes up a large part of the OFC involving both the lateral parts of the medial orbital gyrus as well as the anterior orbital gyrus. The lateral orbital gyrus consists mostly of area 47/12.[14] Most of the OFC is granular, although the caudal parts of area 13 and area 14 are agranular.[16] These caudal regions, which sometimes includes parts of the insular cortex, responds primarily to unprocessed sensory cues.[17]
Connections
The connectivity of the OFC varies somewhat along a rostral-caudal axis. The caudal OFC is more heavily interconnected with sensory regions, notably receiving direct input from the pyriform cortex. The caudal OFC is also the most heavily interconnected with the amygdala.[18] Rostrally, the OFC receives fewer direct sensory projections, and is less connected with the amygdala, but it is interconnected with the lateral prefrontal cortex and parahippocampus.[17] The connectivity of the OFC has also been conceptualized as being composed of two networks; an orbital network composed of most of the central parts of the OFC, including most of areas 47/12, 13, and 11; a medial network composed of the medial most and caudolateral regions of the OFC, as well as areas 24, 25 and 32 of the medial prefrontal cortex.[19] The medial and orbital networks are sometimes referred to as the "visceromotor network" and the "sensory network", respectively.[20]
Afferents
The OFC receives projections from multiple sensory modalities. The primary olfactory cortex, gustatory cortex, secondary somatosensory cortex, superior and inferior temporal gyrus(conveying visual information) all project to the OFC.[16][21][22] Evidence for auditory inputs is weak, although some neurons respond to auditory stimuli, indicating an indirect projection may exist.[19] The OFC also receives input from the medial dorsal nucleus, insular cortex, entorhinal cortex, perirhinal cortex, hypothalamus, and amygdala.[21][23]
Efferents
The orbitofrontal cortex is reciprocally connected with the perirhinal and entorhinal cortices,[23] the amygdala, the hypothalamus, and parts of the medial temporal lobe. In addition to these outputs, the OFC also projects to the striatum, including the nucleus accumbens, caudate nucleus, and ventral putamen, as well as regions of the midbrain including the periaqueductal grey, and ventral tegmental area.[21][24] OFC inputs to the amygdala synapse on multiple targets, including two robust pathways to the basolateral amygdala and intercalated cells of the amygdala, as well as a weaker direct projection to the central nucleus of the amygdala.[18]
Function
Multiple functions have been ascribed to the OFC including mediating context specific responding,[25] encoding contingencies in a flexible manner, encoding value, encoding inferred value, inhibiting responses, learning changes in contingency, emotional appraisal,[26] altering behavior through somatic markers, driving social behavior, and representing state spaces.[27][28] While most of these theories explain certain aspects of electrophysiological observations and lesion related changes in behavior, they often fail to explain, or are contradicted by other findings. One proposal that explains the variety of OFC functions is that the OFC encodes state spaces, or the discrete configuration of internal and external characteristics associated with a situation and its contingencies[29] For example, the proposal that the OFC encodes economic value may be a reflection of the OFC encoding task state value.[25] The representation of task states could also explain the proposal that the OFC acts as a flexible map of contingencies, as a switch in task state would enable the encoding of new contingencies in one state, with the preservation of old contingencies in a separate state, enabling switching contingencies when the old task state becomes relevant again.[28] The representation of task states is supported by electrophysiological evidence demonstrating that the OFC responds to a diverse array of task features, and is capable of rapidly remapping during contingency shifts.[28] The representation of task states may influence behavior through multiple potential mechanisms. For example, the OFC is necessary for ventral tegmental area (VTA) neurons to produce a dopaminergic reward prediction error, and the OFC may encode expectations for computation of RPEs in the VTA.[25]
Specific functions have been ascribed to subregions of the OFC. The lateral OFC has been proposed to reflect potential choice value, enabling fictive(counterfactual) prediction errors to potentially mediate switching choices during reversal, extinction and devaluation.[30] Optogenetic activation of the lOFC enhances goal directed over habitual behavior, possibly reflecting increased sensitivity to potential choices and therefore increased switching. The mOFC, on the other hand, has been proposed to reflect relative subjective value.[26] In rodents, a similar function has been ascribed to the mOFC, encoding action value in a graded fashion, while the lOFC has been proposed to encode specific sensory features of outcomes.[31] The lOFC has also been proposed to encode stimulus outcome associations, which are then compared by value in the mOFC.[32] Meta analysis of neuroimaging studies in humans reveals that a medial-lateral valence gradient exists, with the medial OFC responding most often to reward, and the lateral OFC responding most often to punishment. A posterior-anterior abstractness gradient was also found, with the posterior OFC responding to more simple reward, and the anterior OFC responding more to abstract rewards.[33] Similar results were reported in a meta analysis of studies on primary versus secondary rewards.[34]
The OFC and basolateral amygdala (BLA) are highly interconnected, and their connectivity is necessary for devaluation tasks. Damage to either the BLA or the OFC before, but only the OFC after devaluation impairs performance.[35] While the BLA only responds to cues predicting salient outcomes in a graded fashion in accordance with value, the OFC responds to both value and the specific sensory attributes of cue-outcome associations. While OFC neurons that, early in learning, respond to outcome receipt normally transfer their response to the onset of cues that predict the outcome, damage to the BLA impairs this form of learning.[36]
The posterior orbitofrontal cortex (pOFC) is connected to the amygdala via multiple paths, that are capable of both upregulating and downregulating autonomic nervous system activity.[37] Tentative evidence suggests that the neuromodulator dopamine plays a role in mediating the balance between the inhibitory and excitatory pathways, with a high dopamine state driving autonomic activity.[38]
It has been suggested that the medial OFC is involved in making stimulus-reward associations and with the reinforcement of behavior, while the lateral OFC is involved in stimulus-outcome associations and the evaluation and possibly reversal of behavior.[39] Activity in the lateral OFC is found, for example, when subjects encode new expectations about punishment and social reprisal.[40][41]
The mid-anterior OFC has been found to consistently track subjective pleasure in neuroimaging studies. A hedonic hotspot has been discovered in the anterior OFC, which is capable of enhancing liking response to sucrose. The OFC is also capable of biasing the affective responses induced by α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) antagonism in the nucleus accumbens towards appetitive responses.[42]
The OFC is capable of modulating aggressive behavior via projections to interneurons in the amygdala that inhibit glutaminergic projections to the ventromedial hypothalamus.[43]
Electrophysiology
Neurons in the OFC respond both to primary reinforcers, as well as cues that predict rewards across multiple sensory domains. The evidence for responses to visual, gustatory, somatosensory, and olfactory stimuli is robust, but evidence for auditory responses are weaker. In a subset of OFC neurons, neural responses to rewards or reward cues are modulated by individual preference and by internal motivational states such as hunger. A fraction of neurons that respond to sensory cues predicting a reward are selective for reward, and exhibit reversal behavior when cue outcome relationships are swapped. Neurons in the OFC also exhibit responses to the absence of an expected reward, and punishment. Another population of neurons exhibits responses to novel stimuli and can “remember” familiar stimuli for up to a day.[44]
During cued reward or cued instrumental reward tasks, neurons in the OFC exhibit three general patterns of firing; firing in response to cues; firing before reward receipt; firing in response to reward receipt. In contrast to the medial prefrontal cortex and striatum, OFC neurons do not exhibit firing mediating by movement. Their reward-predictive responses are, however, shaped by attention: when shifting attention between two alternatives, the same OFC population will represent positively the value of a currently attended item, but negatively the value of the unattended item.[45] The encoding of reward magnitude is also flexible, and takes into account the relative values of present rewards.[46]
Humans
The human OFC is among the least-understood regions of the human brain. It has been proposed that the OFC is involved in sensory integration, in representing the affective value of reinforcers, and in decision-making and expectation.[1] In particular, the OFC seems to be important in signaling the expected rewards/punishments of an action given the particular details of a situation.[47] In doing this, the brain is capable of comparing the expected reward/punishment with the actual delivery of reward/punishment, thus, making the OFC critical for adaptive learning. This is supported by research in humans, non-human primates, and rodents.
Psychiatric disorders
The orbitofrontal cortex has been implicated in borderline personality disorder,[48] schizophrenia, major depressive disorder, bipolar disorder, obsessive-compulsive disorder, addiction, post-traumatic stress disorder, Autism,[49] and panic disorder. Although neuroimaging studies have provided evidence for dysfunction in a wide variety of psychiatric disorders, the enigmatic nature of the OFCs role in behavior complicates the understanding of its role in the pathophysiology of psychiatric disorders.[50] The function of the OFC is not known, but its anatomical connections with the ventral striatum, amygdala, hypothalamus, hippocampus, and periaqueductal grey support a role in mediating reward and fear related behaviors.[51]
Obsessive compulsive disorder
Meta analyses of neuroimaging studies in OCD report hyperactivity in areas generally considered to be part of the orbitofrontal segment of the cortico-basal ganglia-thalamo-cortical loop such as the caudate nucleus, thalamus and orbitofrontal cortex. OCD has been proposed to reflect a positive feedback loop due to mutual excitation of the OFC and subcortical structures.[52] While the OFC is usually overactive during symptom provocation tasks, cognitive tasks usually elicit hypoactivity of the OFC;[53] this may reflect a distinction between emotional and non emotional tasks, lateral and medial OFC,[54] or simply just inconsistent methodologies.[55]
Addiction
Animal models, and cell specific manipulations in relation to drug seeking behavior implicate dysfunction of the OFC in addiction.[56] Substance use disorders are associated with a variety of deficits related to flexible goal directed behavior and decision making. These deficits overlap with symptoms related to OFC lesions, and are also associated with reduced orbitofrontal grey matter, resting state hypometabolism, and blunted OFC activity during tasks involving decision making or goal directed behavior. In contrast to resting state and decision related activity, cues associated with drugs evoke robust OFC activity that correlates with craving.[57] Rodent studies also demonstrate that lOFC to BLA projections are necessary for cue induced reinstatement of self administration. These findings are all congruent with the role that the OFC plays in encoding the outcomes associated with certain stimuli.[58][59][60] The progression towards compulsive substance abuse may reflect a shift between model based decision making, where an internal model of future outcomes guides decisions, to model free learning, where decisions are based on reinforcement history. Model based learning involves the OFC and is flexible and goal directed, while model free learning is more rigid; as shift to more model free behavior due to dysfunction in the OFC, like that produced by drugs of misuse, could underlie drug seeking habits.[61]
Behavioral disorders
Conduct disorder is associated with both structural abnormalities, and functional abnormalities during affective tasks.[62] Abnormalities in OFC structure, activity, and functional connectivity have all been observed in association with aggression.[63]
Affective disorders
Neuroimaging studies have found abnormalities in the OFC in MDD and bipolar disorder. Consistent with the medial/reward and lateral/punishment gradient found in neuroimaging studies, some neuroimaging studies have observed elevated lateral OFC activity in depression, as well as reduced interconnectivity of the medial OFC, and enhanced interconnectivity in the lateral OFC.[64] Hypoactivity of the lateral OFC has been frequently observed in bipolar disorder, in particular during manic episodes.[64]
Research
Imaging
Using functional magnetic resonance imaging (fMRI) to image the human OFC is a challenge, because this brain region is in proximity to the air-filled sinuses. This means that artifact errors can occur in the signal processing, causing for example geometric distortions that are common when using echo-planar imaging (EPI) at higher magnetic field strengths. Extra care is therefore recommended for obtaining a good signal from the orbitofrontal cortex, and a number of strategies have been devised, such as automatic shimming at high static magnetic field strengths.[65]
Rodents
In rodents, the OFC is entirely agranular or dysgranular.[16] The OFC is divided into ventrolateral (VLO), lateral (LO), medial (MO) and dorsolateral (DLO) regions.[19] Using highly specific techniques to manipulate circuitry, such as optogenetics, the OFC has been implicated in OCD like behaviors,[66] and in the ability to use latent variables in decision making task.[67]
Clinical significance
Damage
Destruction of the OFC through acquired brain injury typically leads to a pattern of disinhibited behaviour. Examples include swearing excessively, hypersexuality, poor social interaction, compulsive gambling, drug use (including alcohol and tobacco), and poor empathising ability. Disinhibited behaviour by patients with some forms of frontotemporal dementia is thought to be caused by degeneration of the OFC.[68]
Disruption
When OFC connections are disrupted, a number of cognitive, behavioral, and emotional consequences may arise. Research supports that the main disorders associated with dysregulated OFC connectivity/circuitry center around decision-making, emotion regulation, impulsive control, and reward expectation.[69][70][71][72] A recent multi-modal human neuroimaging study shows disrupted structural and functional connectivity of the OFC with the subcortical limbic structures (e.g., amygdala or hippocampus) and other frontal regions (e.g., dorsal prefrontal cortex or anterior cingulate cortex) correlates with abnormal OFC affect (e.g., fear) processing in clinically anxious adults.[73]
One clear extension of problems with decision-making is drug addiction/substance dependence, which can result from disruption of the striato-thalamo-orbitofrontal circuit.[72][70][74] Attention deficit hyperactivity disorder (ADHD) has also been implicated in dysfunction of neural reward circuitry controlling motivation, reward, and impulsivity, including OFC systems.[71] Other disorders of executive functioning and impulse control may be affected by OFC circuitry dysregulation, such as obsessive–compulsive disorder and trichotillomania[75][76][77]
Some dementias are also associated with OFC connectivity disruptions. The behavioral variant of frontotemporal dementia[78] is associated with neural atrophy patterns of white and gray matter projection fibers involved with OFC connectivity.[79] Finally, some research suggests that later stages of Alzheimer's Disease be impacted by altered connectivity of OFC systems.[77]
Orbitofrontal epilepsy
Orbitofrontal epilepsy is rare, but does occur. The presentation of OFC epilepsy is fairly diverse, although common characteristics include sleep related symptoms, automatisms, and hypermotor symptoms. One review reported that auras were generally not common or nonspecific, while another reported that OFC epilepsy was associated auras involving somatosensory phenomenon and fear.[80][81][82]
Assessment
Visual discrimination test
The visual discrimination test has two components. In the first component, "reversal learning", participants are presented with one of two pictures, A and B. They learn that they will be rewarded if they press a button when picture A is displayed, but punished if they press the button when picture B is displayed. Once this rule has been established, the rule swaps. In other words, now it is correct to press the button for picture B, not picture A. Most healthy participants pick up on this rule reversal almost immediately, but patients with OFC damage continue to respond to the original pattern of reinforcement, although they are now being punished for persevering with it. Rolls et al.[83] noted that this pattern of behaviour is particularly unusual given that the patients reported that they understood the rule.
The second component of the test is "extinction". Again, participants learn to press the button for picture A but not picture B. However this time, instead of the rules reversing, the rule changes altogether. Now the participant will be punished for pressing the button in response to either picture. The correct response is not to press the button at all, but people with OFC dysfunction find it difficult to resist the temptation to press the button despite being punished for it.
Iowa Gambling Task
A simulation of real life decision-making, the Iowa gambling task is widely used in cognition and emotion research.[84] Participants are presented with four virtual decks of cards on a computer screen. They are told that each time they choose a card they stand to win some game money. They are told that the aim of the game is to win as much money as possible. Every so often, however, when they choose a card they will lose some money. The task is meant to be opaque, that is, participants are not meant to consciously work out the rule, and they are supposed to choose cards based on their "gut reaction." Two of the decks are "bad decks", which means that, over a long enough time, they will make a net loss; the other two decks are "good decks" and will make a net gain over time.
Most healthy participants sample cards from each deck, and after about 40 or 50 selections are fairly good at sticking to the good decks. Patients with OFC dysfunction, however, continue to perseverate with the bad decks, sometimes even though they know that they are losing money overall. Concurrent measurement of galvanic skin response shows that healthy participants show a "stress" reaction to hovering over the bad decks after only 10 trials, long before conscious sensation that the decks are bad. By contrast, patients with OFC dysfunction never develop this physiological reaction to impending punishment. Bechara and his colleagues explain this in terms of the somatic marker hypothesis. The Iowa gambling task is currently being used by a number of research groups using fMRI to investigate which brain regions are activated by the task in healthy volunteers as well as clinical groups with conditions such as schizophrenia and obsessive compulsive disorder.
The faux pas test is a series of vignettes recounting a social occasion during which someone said something that should not have been said, or an awkward occurrence. The participant's task is to identify what was said that was awkward, why it was awkward, how people would have felt in reaction to the faux pas and to a factual control question. Although first designed for use in people on the autism spectrum,[85] the test is also sensitive to patients with OFC dysfunction, who cannot judge when something socially awkward has happened despite appearing to understand the story perfectly well.
See also
Additional images
 Orbital gyrus shown in red.
Orbital gyrus shown in red.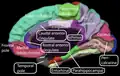 Medial surface of cerebral cortex - gyri
Medial surface of cerebral cortex - gyri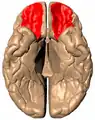 Basal surface of cerebrum. Orbital gyrus shown in red.
Basal surface of cerebrum. Orbital gyrus shown in red.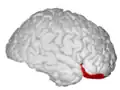 Lateral orbitofrontal cortex
Lateral orbitofrontal cortex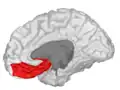 Medial orbitofrontal cortex, inner slice view.
Medial orbitofrontal cortex, inner slice view.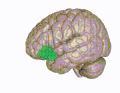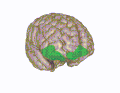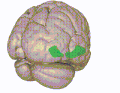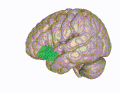 3D visualization of the orbitofrontal cortex in an average human brain
3D visualization of the orbitofrontal cortex in an average human brain Orbitofrontal cortex highlighted in green on coronal T1 MRI images
Orbitofrontal cortex highlighted in green on coronal T1 MRI images Orbitofrontal cortex highlighted in green on sagittal T1 MRI images
Orbitofrontal cortex highlighted in green on sagittal T1 MRI images Orbitofrontal cortex highlighted in green on transversal T1 MRI images
Orbitofrontal cortex highlighted in green on transversal T1 MRI images
References
- 1 2 Kringelbach M. L. (2005). "The orbitofrontal cortex: linking reward to hedonic experience". Nature Reviews Neuroscience. 6 (9): 691–702. doi:10.1038/nrn1747. PMID 16136173. S2CID 205500365.
- ↑ Phillips, LH., MacPherson, SE. & Della Sala, S. (2002). 'Age, cognition and emotion: the role of anatomical segregation in the frontal lobes: the role of anatomical segregation in the frontal lobes'. in J Grafman (ed.), Handbook of Neuropsychology: the frontal lobes. Elsevier Science, Amsterdam, pp. 73-98.
- ↑ Barbas H, Ghashghaei H, Rempel-Clower N, Xiao D (2002) Anatomic basis of functional specialization in prefrontal cortices in primates. In: Handbook of Neuropsychology (Grafman J, ed), pp 1-27. Amsterdam: Elsevier Science B.V.
- ↑ Gottfried JA, Zald DH. On the scent of human olfactory orbitofrontal cortex: meta- analysis and comparison to non-human primates. Brain Res Rev 2005;50:287–304.
- ↑ Rolls ET. The functions of the orbitofrontal cortex. Brain Cogn 2004;55:11–29.
- ↑ Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic expe- rience. Nat Rev Neurosci 2005;6:691–702
- ↑ Rushworth M, Behrens T, Rudebeck P, Walton M. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends Cogn Sci 2007;11: 168–176.
- ↑ Dixon ML, Thiruchselvam R, Todd RM, ChristoffK. Emotion and the prefrontal cortex: an integrative review. Psychol Bull 2017;143:1033–1081.
- ↑ Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog Neurobiol 2004;72: 341–372.
- ↑ Damasio AR. Descartes’Error: Emotion, Reason and the Human Brain. New York: Putnam; 1994.
- ↑ Fuster, J.M. The Prefrontal Cortex, (Raven Press, New York, 1997).
- ↑ Isamah N, Faison W, Payne ME, MacFall J, Steffens DC, Beyer JL, Krishnan R, Taylor WD (2010). "Variability in Frontotemporal Brain Structure: The Importance of Recruitment of African Americans in Neuroscience Research". PLOS ONE. 5 (10): e13642. Bibcode:2010PLoSO...513642I. doi:10.1371/journal.pone.0013642. PMC 2964318. PMID 21049028.
- ↑ Uylings HB, Groenewegen HJ, Kolb B (2003). "Do rats have a prefrontal cortex?". Behav Brain Res. 146 (1–2): 3–17. doi:10.1016/j.bbr.2003.09.028. PMID 14643455. S2CID 32136463.
- 1 2 Mackey, Sott; Petrides, Michael (2006). "Chapter 2: The orbitofrontal cortex: sulcal and gyral morphology and architecture". In Zald, David H.; Rauch, Scott (eds.). The Orbitofrontal Cortex. New York: Oxford University Press. p. 34. ISBN 9780198565741.
- ↑ Mackey, Sott; Petrides, Michael (2006). "Chapter 2: The orbitofrontal cortex: sulcal and gyral morphology and architecture". In Zald, David H.; Rauch, Scott (eds.). The Orbitofrontal Cortex. New York: Oxford University Press. p. 24. ISBN 9780198565741.
- 1 2 3 Passingham, Richard E.; Wise, Steven P. (1012). "Chapter 4 Orbital prefrontal cortex: choosing objects based on outcomes". The Neurobiology of the Prefrontal Cortex: Anatomy, Evolution and Origin of Insight. Great Clarendon Street, Oxford: Oxford University Press. p. 97. ISBN 9780199552917.
- 1 2 Haber, SN; Behrens, TE (3 September 2014). "The neural network underlying incentive-based learning: implications for interpreting circuit disruptions in psychiatric disorders". Neuron. 83 (5): 1019–39. doi:10.1016/j.neuron.2014.08.031. PMC 4255982. PMID 25189208.
- 1 2 Barbas, Helen; Zikopoulos, Basilis (2006). "Chapter 4: Sequential and parallel circuits for emotional processing in the primate orbitofrontal cortex". In Rauch, Scott L.; Zald, David H. (eds.). The Orbitofrontal Cortex. New York: Oxford University Press. p. 67.
- 1 2 3 Price, Joseph L. (2006). "Chapter 3: Connections of the orbital cortex". In Rauch, Scott L.; Zald, David H. (eds.). The Orbitofrontal Cortex. New York: Oxford University Press. p. 42.
- ↑ Rudebeck, PH; Murray, EA (December 2011). "Balkanizing the primate orbitofrontal cortex: distinct subregions for comparing and contrasting values". Annals of the New York Academy of Sciences. 1239 (1): 1–13. Bibcode:2011NYASA1239....1R. doi:10.1111/j.1749-6632.2011.06267.x. PMC 3951748. PMID 22145870.
- 1 2 3 Rolls, ET (March 2000). "The orbitofrontal cortex and reward". Cerebral Cortex. 10 (3): 284–94. doi:10.1093/cercor/10.3.284. PMID 10731223.
- ↑ Rolls, ET (November 2004). "Convergence of sensory systems in the orbitofrontal cortex in primates and brain design for emotion". The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology. 281 (1): 1212–25. doi:10.1002/ar.a.20126. PMID 15470678.
- 1 2 Rempel-Clower, NL (December 2007). "Role of orbitofrontal cortex connections in emotion". Annals of the New York Academy of Sciences. 1121 (1): 72–86. Bibcode:2007NYASA1121...72R. doi:10.1196/annals.1401.026. PMID 17846152. S2CID 21317263.
- ↑ Price, Joseph L. (2006). "Chapter 3: Connections of the orbital cortex". In Rauch, Scott L.; Zald, David H. (eds.). The Orbitofrontal Cortex. New York: Oxford University Press. p. 45.
- 1 2 3 Wikenheiser, AM; Schoenbaum, G (August 2016). "Over the river, through the woods: cognitive maps in the hippocampus and orbitofrontal cortex". Nature Reviews. Neuroscience. 17 (8): 513–23. doi:10.1038/nrn.2016.56. PMC 5541258. PMID 27256552.
- 1 2 Fettes, P; Schulze, L; Downar, J (2017). "Cortico-Striatal-Thalamic Loop Circuits of the Orbitofrontal Cortex: Promising Therapeutic Targets in Psychiatric Illness". Frontiers in Systems Neuroscience. 11: 25. doi:10.3389/fnsys.2017.00025. PMC 5406748. PMID 28496402.
- ↑ Wilson, Robert C.; Takahashi, Yuji K.; Schoenbaum, Geoffrey; Niv, Yael (January 2014). "Orbitofrontal Cortex as a Cognitive Map of Task Space". Neuron. 81 (2): 267–279. doi:10.1016/j.neuron.2013.11.005. ISSN 0896-6273. PMC 4001869. PMID 24462094.
- 1 2 3 Sadacca, BF; Wikenheiser, AM; Schoenbaum, G (14 March 2017). "Toward a theoretical role for tonic norepinephrine in the orbitofrontal cortex in facilitating flexible learning". Neuroscience. 345: 124–129. doi:10.1016/j.neuroscience.2016.04.017. PMC 5461826. PMID 27102419.
- ↑ Stalnaker, TA; Cooch, NK; Schoenbaum, G (May 2015). "What the orbitofrontal cortex does not do". Nature Neuroscience. 18 (5): 620–7. doi:10.1038/nn.3982. PMC 5541252. PMID 25919962.
- ↑ Tobia, M. J.; Guo, R.; Schwarze, U.; Boehmer, W.; Gläscher, J.; Finckh, B.; Marschner, A.; Büchel, C.; Obermayer, K.; Sommer, T. (2014-04-01). "Neural systems for choice and valuation with counterfactual learning signals". NeuroImage. 89: 57–69. doi:10.1016/j.neuroimage.2013.11.051. ISSN 1053-8119. PMID 24321554. S2CID 35280557.
- ↑ Izquierdo, A (1 November 2017). "Functional Heterogeneity within Rat Orbitofrontal Cortex in Reward Learning and Decision Making". The Journal of Neuroscience. 37 (44): 10529–10540. doi:10.1523/JNEUROSCI.1678-17.2017. PMC 6596524. PMID 29093055.
- ↑ Rudebeck, PH; Murray, EA (December 2011). "Balkanizing the primate orbitofrontal cortex: distinct subregions for comparing and contrasting values". Annals of the New York Academy of Sciences. 1239 (1): 1–13. Bibcode:2011NYASA1239....1R. doi:10.1111/j.1749-6632.2011.06267.x. PMC 3951748. PMID 22145870.
- ↑ Kringelbach, ML; Rolls, ET (April 2004). "The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology". Progress in Neurobiology. 72 (5): 341–72. doi:10.1016/j.pneurobio.2004.03.006. PMID 15157726. S2CID 13624163.
- ↑ Sescousse, G; Caldú, X; Segura, B; Dreher, JC (May 2013). "Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies". Neuroscience and Biobehavioral Reviews. 37 (4): 681–96. doi:10.1016/j.neubiorev.2013.02.002. hdl:2066/117487. PMID 23415703. S2CID 8335094.
- ↑ Padoa-Schioppa, C; Conen, KE (15 November 2017). "Orbitofrontal Cortex: A Neural Circuit for Economic Decisions". Neuron. 96 (4): 736–754. doi:10.1016/j.neuron.2017.09.031. PMC 5726577. PMID 29144973.
- ↑ Sharpe, MJ; Schoenbaum, G (May 2016). "Back to basics: Making predictions in the orbitofrontal-amygdala circuit". Neurobiology of Learning and Memory. 131: 201–6. doi:10.1016/j.nlm.2016.04.009. PMC 5541254. PMID 27112314.
- ↑ Barbas, H (August 2007). "Flow of information for emotions through temporal and orbitofrontal pathways". Journal of Anatomy. 211 (2): 237–49. doi:10.1111/j.1469-7580.2007.00777.x. PMC 2375774. PMID 17635630.
The posterior orbitofrontal cortex targets dual systems in the amygdala which have opposite effects on central autonomic structures. Both pathways originate in posterior orbitofrontal cortex, but one targets heavily the inhibitory intercalated masses, whose activation can ultimately disinhibit central autonomic structures during emotional arousal.
- ↑ Zikopoulos, B; Höistad, M; John, Y; Barbas, H (17 May 2017). "Posterior Orbitofrontal and Anterior Cingulate Pathways to the Amygdala Target Inhibitory and Excitatory Systems with Opposite Functions". The Journal of Neuroscience. 37 (20): 5051–5064. doi:10.1523/JNEUROSCI.3940-16.2017. PMC 5444191. PMID 28411274.
The specific innervation of inhibitory systems in the amygdala found here, along with the differential impact that dopamine has on them, makes it possible to hypothesize how distinct autonomic states may be achieved. A strong pOFC influence on IM that activates DARPP-32+ and CB+ neurons may help modulate autonomic function by downregulating CeM and thereby facilitate social interactions in primates....On the other hand, in a panic condition, when survival is perceived to be threatened, dopamine levels markedly increase. DARPP-32+ neurons in IM may thus be primarily inhibited, rendering the pOFC pathway ineffective.
- ↑ Walton M. E.; Behrens T. E.; Buckley M. J.; Rudebeck P. H.; Rushworth M. F. (2010). "Separable learning systems in the macaque brain and the role of orbitofrontal cortex in contingent learning". Neuron. 65 (6): 927–939. doi:10.1016/j.neuron.2010.02.027. PMC 3566584. PMID 20346766.
- ↑ Campbell-Meiklejohn D. K.; Kanai R.; Bahrami B.; Bach D. R.; Dolan R. J.; Roepstorff A.; Frith C. D. (2012). "Structure of orbitofrontal cortex predicts social influence". Current Biology. 22 (4): R123–R124. doi:10.1016/j.cub.2012.01.012. PMC 3315000. PMID 22361146.
- ↑ Tanferna A.; López-Jiménez L.; Blas J.; Hiraldo F.; Sergio F. (2012). "How Expert Advice Influences Decision Making". PLOS ONE. 7 (11): e49748. Bibcode:2012PLoSO...749748M. doi:10.1371/journal.pone.0049748. PMC 3504100. PMID 23185425.
- ↑ Berridge, KC; Kringelbach, ML (6 May 2015). "Pleasure systems in the brain". Neuron. 86 (3): 646–64. doi:10.1016/j.neuron.2015.02.018. PMC 4425246. PMID 25950633.
- ↑ Numan, Michael (2015). Neurobiology of Social Behavior: Toward an Understanding of the Prosocial and Antisocial Brain. Londong: Elsevier Science. p. 85.
- ↑ Rolls, Edmund T. (2006). "Chapter 5 The Neurophysiology and Functions of the Orbitofrontal Cortex". In Zald, David H.; Rauch, Scott L. (eds.). The Orbitofrontal Cortex. New York: Oxford University Press.
- ↑ Hunt LT; Malalasekera WMN; de Berker AO; Miranda B; Farmer S; Behrens TEJ; Kennerley SW (26 September 2018). "Triple dissociation of attention and decision computations across prefrontal cortex". Nature Neuroscience. 21 (9): 1471–1481. doi:10.1038/s41593-018-0239-5. PMC 6331040. PMID 30258238.
- ↑ Schultz, Wolfram; Tremblay, Leon (2006). "Chapter 7: Involvement of primate orbitofrontal neurons in reward, uncertainty, and learning 173 Wolfram Schultz and Leon Tremblay". In Zald, David H.; Rauch, Scott :L. (eds.). The Orbitofrontal Cortex. New York: Oxford University Press.
- ↑ Schoenbaum G, Takahashi Y, Liu T, McDannald M (2011). "Does the orbitofrontal cortex signal value?". Annals of the New York Academy of Sciences. 1239 (1): 87–99. Bibcode:2011NYASA1239...87S. doi:10.1111/j.1749-6632.2011.06210.x. PMC 3530400. PMID 22145878.
- ↑ Berlin, HA; Rolls, ET; Iversen, SD (October 2005). "Borderline Personality Disorder, Impulsivity, and the Orbitofrontal Cortex". Archives of Clinical Neuropsychology. 20 (7): 862–863.
- ↑ Ha, Sungji; Sohn, In-Jung; Kim, Namwook; Sim, Hyeon Jeong; Cheon, Keun-Ah (December 2015). "Characteristics of Brains in Autism Spectrum Disorder: Structure, Function and Connectivity across the Lifespan". Experimental Neurobiology. 24 (4): 273–284. doi:10.5607/en.2015.24.4.273. ISSN 1226-2560. PMC 4688328. PMID 26713076.
- ↑ Jackowski, AP; Araújo Filho, GM; Almeida, AG; Araújo, CM; Reis, M; Nery, F; Batista, IR; Silva, I; Lacerda, AL (June 2012). "The involvement of the orbitofrontal cortex in psychiatric disorders: an update of neuroimaging findings". Revista Brasileira de Psiquiatria. 34 (2): 207–12. doi:10.1590/S1516-44462012000200014. PMID 22729418.
- ↑ Milad, MR; Rauch, SL (December 2007). "The role of the orbitofrontal cortex in anxiety disorders". Annals of the New York Academy of Sciences. 1121 (1): 546–61. Bibcode:2007NYASA1121..546M. doi:10.1196/annals.1401.006. PMID 17698998. S2CID 34467365.
- ↑ Nakao, T; Okada, K; Kanba, S (August 2014). "Neurobiological model of obsessive-compulsive disorder: evidence from recent neuropsychological and neuroimaging findings". Psychiatry and Clinical Neurosciences. 68 (8): 587–605. doi:10.1111/pcn.12195. PMID 24762196. S2CID 5528241.
- ↑ Fineberg, NA; Potenza, MN; Chamberlain, SR; Berlin, HA; Menzies, L; Bechara, A; Sahakian, BJ; Robbins, TW; Bullmore, ET; Hollander, E (February 2010). "Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review". Neuropsychopharmacology. 35 (3): 591–604. doi:10.1038/npp.2009.185. PMC 3055606. PMID 19940844.
- ↑ Milad, MR; Rauch, SL (January 2012). "Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways". Trends in Cognitive Sciences. 16 (1): 43–51. doi:10.1016/j.tics.2011.11.003. PMC 4955838. PMID 22138231.
- ↑ Vaghi, M; Robbins, T. "TASK-BASED FUNCTIONAL NEUROIMAGING STUDIES OF OBSESSIVE-COMPULSIVE DISORDER: A HYPOTHESIS-DRIVEN REVIEW". In Pittenger, Christopher (ed.). Obsessive Compulsive Disorder, Phenomenology, Pathophysiology and Treatment. Oxford University Press. pp. 239–240.
- ↑ Schoenbaum, G; Chang, CY; Lucantonio, F; Takahashi, YK (December 2016). "Thinking Outside the Box: Orbitofrontal Cortex, Imagination, and How We Can Treat Addiction". Neuropsychopharmacology. 41 (13): 2966–2976. doi:10.1038/npp.2016.147. PMC 5101562. PMID 27510424.
- ↑ Koob, GF; Volkow, ND (January 2010). "Neurocircuitry of addiction". Neuropsychopharmacology. 35 (1): 217–38. doi:10.1038/npp.2009.110. PMC 2805560. PMID 19710631.
- ↑ Moorman, DE (2 February 2018). "The role of the orbitofrontal cortex in alcohol use, abuse, and dependence". Progress in Neuro-psychopharmacology & Biological Psychiatry. 87 (Pt A): 85–107. doi:10.1016/j.pnpbp.2018.01.010. PMC 6072631. PMID 29355587.
- ↑ Gowin, JL; Mackey, S; Paulus, MP (1 September 2013). "Altered risk-related processing in substance users: imbalance of pain and gain". Drug and Alcohol Dependence. 132 (1–2): 13–21. doi:10.1016/j.drugalcdep.2013.03.019. PMC 3748224. PMID 23623507.
Individuals with SUDs show several processing abnormalities during risk-taking decision-making, which include altered valuation of options (VMPFC) and outcomes (OFC and striatum), poor estimation of uncertainty (ACC and insular cortex), diminished executive control (DLPFC), and an attenuated influence of emotional salience (amygdala), and reduced responsiveness to somatic markers (somatosensory cortex). These neural processing differences during risk-taking among individuals with SUDs have been linked to poorer behavioral performance on risk-taking tasks and a more extensive history of substance use
- ↑ Chase, HW; Eickhoff, SB; Laird, AR; Hogarth, L (15 October 2011). "The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis". Biological Psychiatry. 70 (8): 785–93. doi:10.1016/j.biopsych.2011.05.025. PMC 4827617. PMID 21757184.
A medial region of the OFC showed greater activation by drug cues compared with control cues and was consistently activated in the nontreatment-seeking subgroup. There is substantial evidence that this region plays a role in appetitive behavior and decision making (86,87), in particular with regard to expectations of reward (88) predicted by conditioned stimuli (89–94), which can control instrumental action selectio
- ↑ Lucantonio, F; Caprioli, D; Schoenbaum, G (January 2014). "Transition from 'model-based' to 'model-free' behavioral control in addiction: Involvement of the orbitofrontal cortex and dorsolateral striatum". Neuropharmacology. 76 Pt B: 407–15. doi:10.1016/j.neuropharm.2013.05.033. PMC 3809026. PMID 23752095.
- ↑ Rubia, K (15 June 2011). ""Cool" inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus "hot" ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review". Biological Psychiatry. 69 (12): e69–87. doi:10.1016/j.biopsych.2010.09.023. PMID 21094938. S2CID 14987165.
- ↑ Rosell, DR; Siever, LJ (June 2015). "The neurobiology of aggression and violence". CNS Spectrums. 20 (3): 254–79. doi:10.1017/S109285291500019X. PMID 25936249.
- 1 2 Rolls, ET (September 2016). "A non-reward attractor theory of depression" (PDF). Neuroscience and Biobehavioral Reviews. 68: 47–58. doi:10.1016/j.neubiorev.2016.05.007. PMID 27181908. S2CID 8145667.
- ↑ J. Wilson; M. Jenkinson; I. E. T. de Araujo; Morten L. Kringelbach; E. T. Rolls & Peter Jezzard (October 2002). "Fast, fully automated global and local magnetic field optimization for fMRI of the human brain". NeuroImage. 17 (2): 967–976. doi:10.1016/S1053-8119(02)91172-9. PMID 12377170.
- ↑ Ahmari, SE; Dougherty, DD (August 2015). "Dissecting Ocd Circuits: From Animal Models to Targeted Treatments". Depression and Anxiety. 32 (8): 550–62. doi:10.1002/da.22367. PMC 4515165. PMID 25952989.
- ↑ Vertechi, Pietro; Lottem, Eran; Sarra, Dario; Godinho, Beatriz; Treves, Isaac; Quendera, Tiago; Lohuis, Matthijs Nicolai Oude; Mainen, Zachary F. (2020-04-08). "Inference-Based Decisions in a Hidden State Foraging Task: Differential Contributions of Prefrontal Cortical Areas". Neuron. 106 (1): 166–176.e6. doi:10.1016/j.neuron.2020.01.017. ISSN 0896-6273. PMC 7146546. PMID 32048995.
- ↑ Snowden J. S.; Bathgate D.; Varma A.; Blackshaw A.; Gibbons Z. C.; Neary D. (2001). "Distinct behavioural profiles in frontotemporal dementia and semantic dementia". J Neurol Neurosurg Psychiatry. 70 (3): 323–332. doi:10.1136/jnnp.70.3.323. PMC 1737271. PMID 11181853.
- ↑ Xiao, Xiong; Deng, Hanfei; Wei, Lei; Huang, Yanwang; Wang, Zuoren (September 2016). "Neural activity of orbitofrontal cortex contributes to control of waiting". The European Journal of Neuroscience. 44 (6): 2300–2313. doi:10.1111/ejn.13320. ISSN 1460-9568. PMID 27336203. S2CID 205105682.
- 1 2 Paulus M. P.; Hozack N. E.; Zauscher B. E.; Frank L.; Brown G. G.; Braff D. L.; Schuckit M. A. (2002). "Behavioral and Functional Neuroimaging Evidence for Prefrontal Dysfunction in Methamphetamine-Dependent Subjects". Neuropsychopharmacology. 26 (1): 53–63. doi:10.1016/s0893-133x(01)00334-7. PMID 11751032.
- 1 2 Toplak M. E.; Jain U.; Tannock R. (2005). "Executive and motivational processes in adolescents with Attention-Deficit-Hyperactivity Disorder (ADHD)". Behavioral and Brain Functions. 1 (1): 8–20. doi:10.1186/1744-9081-1-8. PMC 1183187. PMID 15982413.
- 1 2 Verdejo-Garcia A.; Bechara A.; Recknor E. C.; Perez-Garcia M. (2006). "Executive dysfunction in substance dependent individuals during drug use and abstinence: An examination of the behavioral, cognitive and emotional correlates of addiction". Journal of the International Neuropsychological Society. 12 (3): 405–415. doi:10.1017/s1355617706060486. PMID 16903133. S2CID 15939155.
- ↑ Cha, Jiook; Greenberg, Tsafrir; Carlson, Joshua M.; DeDora, Daniel J.; Hajcak, Greg; Mujica-Parodi, Lilianne R. (2014-03-12). "Circuit-Wide Structural and Functional Measures Predict Ventromedial Prefrontal Cortex Fear Generalization: Implications for Generalized Anxiety Disorder". The Journal of Neuroscience. 34 (11): 4043–4053. doi:10.1523/JNEUROSCI.3372-13.2014. ISSN 0270-6474. PMC 6705282. PMID 24623781.
- ↑ Volkow N.D.; Fowler J.S. (2000). "Addiction a disease of compulsion and drive: involvement of the orbitofrontal cortex". Cerebral Cortex. 10 (3): 318–325. doi:10.1093/cercor/10.3.318. PMID 10731226.
- ↑ Chamberlain S. R.; Odlaug B. L.; Boulougouris V.; Fineberg N. A.; Grant J. E. (2009). "Trichotillomania: Neurobiology and treatment". Neuroscience and Biobehavioral Reviews. 33 (6): 831–842. doi:10.1016/j.neubiorev.2009.02.002. PMID 19428495. S2CID 6956143.
- ↑ Menzies L. (2008). "Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: The orbitofronto-striatal model revisited". Neuroscience and Biobehavioral Reviews. 32 (3): 525–549. doi:10.1016/j.neubiorev.2007.09.005. PMC 2889493. PMID 18061263.
- 1 2 Tekin S.; Cummings J. L. (2002). "Frontal-subcortical neuronal circuits and clinical neuropsychiatry: An update". Journal of Psychosomatic Research. 53 (2): 647–654. doi:10.1016/s0022-3999(02)00428-2. PMID 12169339.
- ↑ Rahman S.; Sahakian B. J.; Hodges J. R.; Rogers R. D.; Robbins T. W. (1999). "Specific cognitive deficits in early behavioural variant frontotemporal dementia". Brain. 122 (8): 1469–1493. doi:10.1093/brain/122.8.1469. PMID 10430832.
- ↑ Seeley W. W.; Crawford R.; Rascovsky K.; Kramer J. H.; Weiner M.; Miller B. L.; Gorno-Tempini L. (2008). "Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia". Archives of Neurology. 65 (2): 249–255. doi:10.1001/archneurol.2007.38. PMC 2544627. PMID 18268196.
- ↑ Chibane, IS; Boucher, O; Dubeau, F; Tran, TPY; Mohamed, I; McLachlan, R; Sadler, RM; Desbiens, R; Carmant, L; Nguyen, DK (November 2017). "Orbitofrontal epilepsy: Case series and review of literature". Epilepsy & Behavior. 76: 32–38. doi:10.1016/j.yebeh.2017.08.038. PMID 28928072. S2CID 13656956.
- ↑ Gold, JA; Sher, Y; Maldonado, JR (2016). "Frontal Lobe Epilepsy: A Primer for Psychiatrists and a Systematic Review of Psychiatric Manifestations". Psychosomatics. 57 (5): 445–64. doi:10.1016/j.psym.2016.05.005. PMID 27494984.
- ↑ Smith, JR; Sillay, K; Winkler, P; King, DW; Loring, DW (2004). "Orbitofrontal epilepsy: electroclinical analysis of surgical cases and literature review". Stereotactic and Functional Neurosurgery. 82 (1): 20–5. doi:10.1159/000076656. PMID 15007215. S2CID 18811550.
- ↑ Rolls E. T.; Hornak J.; Wade D.; McGrath J. (1994). "Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage". J Neurol Neurosurg Psychiatry. 57 (12): 1518–1524. doi:10.1136/jnnp.57.12.1518. PMC 1073235. PMID 7798983.
- ↑ Bechara A.; Damasio A. R.; Damasio H.; Anderson S.W. (1994). "Insensitivity to future consequences following damage to human prefrontal cortex". Cognition. 50 (1–3): 7–15. doi:10.1016/0010-0277(94)90018-3. PMID 8039375. S2CID 204981454.
- ↑ Stone V.E.; Baron-Cohen S.; Knight R. T. (1998a). "Frontal Lobe Contributions to Theory of Mind". Journal of Medical Investigation. 10 (5): 640–656. CiteSeerX 10.1.1.330.1488. doi:10.1162/089892998562942. PMID 9802997. S2CID 207724498.
External links
| Wikimedia Commons has media related to Orbitofrontal cortex. |
- Cerebral Cortex special issue on orbitofrontal cortex
- Camille et al. (2004) The Involvement of the Orbitofrontal Cortex in the Experience of Regret