Leuprorelin
 | |
 | |
| Names | |
|---|---|
| Trade names | Lupron, Eligard, Lucrin, others |
| Other names | Leuprolide; Leuprolidine; A-43818; Abbott-43818; DC-2-269; TAP-144 |
IUPAC name
| |
| Clinical data | |
| Drug class | GnRH analogue; GnRH agonist; Antigonadotropin |
| Main uses | Prostate cancer, breast cancer, endometriosis, uterine fibroids, early puberty[1][2] |
| Side effects | Hot flashes, unstable mood, trouble sleeping, headaches, pain at the site of injection[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | implant, injection |
| Defined daily dose | 60 mcg (implant) 1 mg (parenteral) 0.13 mg (depot injection)[3] |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a685040 |
| Legal | |
| Legal status |
|
| Pharmacokinetics | |
| Elimination half-life | 3 hours |
| Excretion | Kidney |
| Chemical and physical data | |
| Formula | C59H84N16O12 |
| Molar mass | 1209.421 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Leuprorelin, also known as leuprolide, is a manufactured version of a hormone used to treat prostate cancer, breast cancer, endometriosis, uterine fibroids, and early puberty.[1][2] It is given by injection into a muscle or under the skin.[1]
Common side effects include hot flashes, unstable mood, trouble sleeping, headaches, and pain at the site of injection.[1] Other side effects may include high blood sugar, allergic reactions, and problems with the pituitary gland.[1] Use during pregnancy may harm the baby.[1] Leuprorelin is in the gonadotropin-releasing hormone (GnRH) analogue family of medications.[1] It works by decreasing gonadotropin and therefore decreasing testosterone and estradiol.[1]
Leuprorelin was patented in 1973 and approved for medical use in the United States in 1985.[1][4] It is on the World Health Organization's List of Essential Medicines.[5] In the United Kingdom a monthly dose costs the NHS about GB£75.24.[6] In the United States the equivalent dose has a wholesale cost of US$1,011.93.[7] It is sold under the brand name Lupron among others.[1]
Medical use
Leuprorelin may be used in the treatment of hormone-responsive cancers such as prostate cancer and breast cancer. It may also be used for estrogen-dependent conditions such as endometriosis[8] or uterine fibroids.
It may be used for precocious puberty in both males and females,[9] and to prevent premature ovulation in cycles of controlled ovarian stimulation for in vitro fertilization (IVF).
It may be used to reduce the risk of premature ovarian failure in women receiving cyclophosphamide for chemotherapy.[10]
Along with triptorelin and goserelin, it has been used to delay puberty in transgender youth until they are old enough to begin hormone replacement therapy.[11] Researchers have recommended puberty blockers after age 12, when the person has developed to Tanner stages 2–3, and then cross-sex hormones treatment at age 16. This use of the drug is off-label, however, not having been approved by the Food and Drug Administration and without data on long-term effects of this use.[12]
They are also sometimes used as alternatives to antiandrogens like spironolactone and cyproterone acetate for suppressing testosterone production in transgender women.
It is considered a possible treatment for paraphilias.[13] Leuprorelin has been tested as a treatment for reducing sexual urges in pedophiles and other cases of paraphilia.[14][15]
Dosage
The defined daily dose is 60 mcg (implant) or 1 mg (parenteral) or 0.13 mg (depot injection)[3]
Side effects
Common side effects of leuprorelin injection include redness/burning/stinging/pain/bruising at the injection site, hot flashes (flushing), increased sweating, night sweats, tiredness, headache, upset stomach, nausea, diarrhea, constipation, stomach pain, breast swelling or tenderness, acne, joint/muscle aches or pain, trouble sleeping (insomnia), reduced sexual interest, vaginal discomfort/dryness/itching/discharge, vaginal bleeding, swelling of the ankles/feet, increased urination at night, dizziness, breakthrough bleeding in a female child during the first 2 months of leuprorelin treatment, weakness, chills, clammy skin, skin redness, itching, or scaling, testicle pain, impotence, depression, or memory problems.[16] The rates of gynecomastia with leuprorelin have been found to range from 3 to 16%.[17]
Pharmacology
Mechanism of action
Leuprorelin is a gonadotropin-releasing hormone (GnRH) analogue acting as an agonist at pituitary GnRH receptors. Agonism of GnRH receptors initially results in the stimulation of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) secretion by the anterior pituitary ultimately leading to increased serum estradiol and testosterone levels via the normal physiology of the hypothalamic–pituitary–gonadal axis (HPG axis); however, because propagation of the HPG axis is incumbent upon pulsatile hypothalamic GnRH secretion, pituitary GnRH receptors become desensitised after several weeks of continuous leuprorelin therapy. This protracted downregulation of GnRH receptor activity is the targeted objective of leuprorelin therapy and ultimately results in decreased LH and FSH secretion, leading to hypogonadism and thus a dramatic reduction in estradiol and testosterone levels regardless of sex.[18][19]
In the treatment of prostate cancer, the initial increase in testosterone levels associated with the initiation of leuprorelin therapy is counterproductive to treatment goals. This effect is avoided with concurrent utilisation of 5α-reductase inhibitors, such as finasteride, which function to block the downstream effects of testosterone.
Chemistry
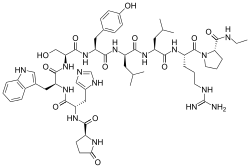
The peptide sequence is Pyr-His-Trp-Ser-Tyr-D-Leu-Leu-Arg-Pro-NHEt (Pyr = L-Pyroglutamyl).
History
Leuprorelin was discovered and first patented in 1973 and was introduced for medical use in 1985.[20][21] It was initially marketed only for daily injection, but a depot injection formulation was introduced in 1989.[21]
Society and culture
Names
Leuprorelin is the generic name of the drug and its INN and BAN, while leuprorelin acetate is its BANM and JAN, leuprolide acetate is its USAN and USP, leuprorelina is its DCIT, and leuproréline is its DCF.[22][23][24][25] It is also known by its developmental code names A-43818, Abbott-43818, DC-2-269, and TAP-144.[22][23][24][25]
Leuprorelin is marketed by Bayer AG under the brand name Viadur, by Tolmar under the brand name Eligard, and by TAP Pharmaceuticals (1985–2008), by Varian Darou Pajooh under the brand name Leupromer and Abbott Laboratories (2008–present) under the brand name Lupron. It is available as a slow-release implant or subcutaneous/intramuscular injection.
In the UK and Ireland, leuprorelin is marketed by Takeda UK as Prostap SR (one-month injection) and Prostap 3 (three-month injection).
Available forms
Leuprorelin is available in the following forms, among others:[26][27][28]
- Short-acting daily intramuscular injection (Lupron): 5 mg/mL (2.8 mL) used as 1 mg every day.
- Long-acting depot intramuscular injection (Lupron Depot): 7.5 mg once a month, 22.5 mg every 3 months, or 30 mg every 4 months.
- Long-acting depot subcutaneous injection (Eligard): 7.5 mg once a month, 22.5 mg every 3 months, 30 mg every 4 months, or 45 mg every 6 months.
- Long-acting subcutaneous implant (Viadur): 65 mg pellet once every 12 months.
Approvals
- Lupron injection was first approved by the FDA for treatment of advanced prostate cancer on April 9, 1985.
- Lupron depot for monthly intramuscular injection was first approved by the FDA for palliative treatment of advanced prostate cancer on January 26, 1989, and subsequently in 22.5 mg/vial and 30 mg/vial for intramuscular depot injection every 3 and 4 months, respectively. 3.75 mg/vial and 11.25 mg/vial dosage forms were subsequently approved for subcutaneous depot injection every month and every 3 months, respectively for treatment of endometriosis or fibroids. 7.5 mg/vial, 11.25 mg/vial, and 15 mg/vial dosage forms were subsequently approved for subcutaneous depot injection for treatment of children with central precocious puberty.
- Viadur (72 mg yearly subcutaneous implant) was first approved by the FDA for palliative treatment of advanced prostate cancer on March 6, 2000. Bayer will fulfill orders until current supplies are depleted, expected by the end of April 2008
- Eligard (7.5 mg for monthly subcutaneous depot injection) was first approved by the FDA for palliative treatment of advanced prostate cancer on January 24, 2002, and subsequently in 22.5 mg, 30 mg, and 45 mg doses for subcutaneous depot injection every 3, 4, and 6 months, respectively.
- Leupromer 7.5 (7.5 mg, one month depot for subcutaneous injection) is the second in situ-forming injectable drug in the world. It is used for palliative treatment of advanced prostate cancer, endometriosis, and uterine fibroids. It was approved by The Ministry of Health and Medical Education Of Iran.
"Lupron protocol"
A 2005 paper in the controversial and non-peer reviewed journal Medical Hypotheses suggested leuprorelin as a possible treatment for autism,[29] the hypothetical method of action being the now defunct hypothesis that autism is caused by mercury, with the additional unfounded assumption that mercury binds irreversibly to testosterone and therefore leuprorelin can help cure autism by lowering the testosterone levels and thereby mercury levels.[30] However, there is no scientifically valid or reliable research to show its effectiveness in treating autism.[31] This use has been termed the "Lupron protocol"[32] and Mark Geier, the proponent of the hypothesis, has frequently been barred from testifying in vaccine-autism related cases on the grounds of not being sufficiently expert in that particular issue[33][34][35] and has had his medical license revoked.[32] Medical experts have referred to Geier's claims as "junk science".[36]
Veterinary use
Leuprorelin is frequently used in ferrets for the treatment of adrenal disease. Its use has been reported in a ferret with concurrent primary hyperaldosteronism,[37] and one with concurrent diabetes mellitus.[38]
Research
As of 2006 leuprorelin was under investigation for possible use in the treatment of mild to moderate Alzheimer's disease.[39]
A by mouth formulation of leuprorelin is under development for the treatment of endometriosis.[40] It was also under development for the treatment of precocious puberty, prostate cancer, and uterine fibroids, but development for these uses was discontinued.[40] The formulation has the tentative brand name Ovarest.[40] As of July 2018, it is in phase II clinical trials for endometriosis.[40]
See also
- Gonadotropin-releasing hormone receptor § Agonists
References
- 1 2 3 4 5 6 7 8 9 10 11 "Leuprolide Acetate". The American Society of Health-System Pharmacists. Archived from the original on 23 December 2016. Retrieved 8 December 2016.
- 1 2 "19th WHO Model List of Essential Medicines (April 2015)" (PDF). WHO. April 2015. Archived (PDF) from the original on May 13, 2015. Retrieved May 10, 2015.
- 1 2 "WHOCC - ATC/DDD Index". www.whocc.no. Archived from the original on 10 April 2021. Retrieved 17 September 2020.
- ↑ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 514. ISBN 9783527607495. Archived from the original on 2019-03-06. Retrieved 2019-03-02.
- ↑ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 655. ISBN 9780857111562.
- ↑ "NADAC as of 2016-12-07 | Data.Medicaid.gov". Centers for Medicare and Medicaid Services. Archived from the original on 21 December 2016. Retrieved 23 December 2016.
- ↑ Crosignani PG, Luciano A, Ray A, Bergqvist A (January 2006). "Subcutaneous depot medroxyprogesterone acetate versus leuprolide acetate in the treatment of endometriosis-associated pain". Human Reproduction. 21 (1): 248–56. doi:10.1093/humrep/dei290. PMID 16176939.
- ↑ Badaru A, Wilson DM, Bachrach LK, et al. (May 2006). "Sequential comparisons of one-month and three-month depot leuprolide regimens in central precocious puberty". The Journal of Clinical Endocrinology and Metabolism. 91 (5): 1862–7. doi:10.1210/jc.2005-1500. PMID 16449344.
- ↑ Clowse ME, Behera MA, Anders CK, Copland S, Coffman CJ, Leppert PC, Bastian LA (March 2009). "Ovarian preservation by GnRH agonists during chemotherapy: a meta-analysis". Journal of Women's Health. 18 (3): 311–9. doi:10.1089/jwh.2008.0857. PMC 2858300. PMID 19281314.
- ↑ David A. Wolfe; Eric J. Mash (9 October 2008). Behavioral and Emotional Disorders in Adolescents: Nature, Assessment, and Treatment. Guilford Press. pp. 556–. ISBN 978-1-60623-115-9. Archived from the original on 2 July 2014. Retrieved 24 March 2012.
- ↑ Dreger, A. (2009, Jan.-Feb.). Gender Identity Disorder in childhood: Inconclusive advice to parents. Hastings Center Report, pp. 26-29.
- ↑ Saleh FM, Niel T, Fishman MJ (2004). "Treatment of paraphilia in young adults with leuprolide acetate: a preliminary case report series". Journal of Forensic Sciences. 49 (6): 1343–8. doi:10.1520/JFS2003035. PMID 15568711.
- ↑ Schober JM, Byrne PM, Kuhn PJ (2006). "Leuprolide acetate is a familiar drug that may modify sex-offender behaviour: the urologist's role". BJU International. 97 (4): 684–6. doi:10.1111/j.1464-410X.2006.05975.x. PMID 16536753.
- ↑ Schober JM, Kuhn PJ, Kovacs PG, Earle JH, Byrne PM, Fries RA (2005). "Leuprolide acetate suppresses pedophilic urges and arousability". Archives of Sexual Behavior. 34 (6): 691–705. doi:10.1007/s10508-005-7929-2. PMID 16362253.
- ↑ "Common Side Effects of Lupron (Leuprolide Acetate Injection) Drug Center". Archived from the original on 2015-07-29. Retrieved 2015-07-26.
- ↑ Di Lorenzo G, Autorino R, Perdonà S, De Placido S (December 2005). "Management of gynaecomastia in patients with prostate cancer: a systematic review". Lancet Oncol. 6 (12): 972–9. doi:10.1016/S1470-2045(05)70464-2. PMID 16321765.
- ↑ Mutschler, Ernst; Schäfer-Korting, Monika (2001). Arzneimittelwirkungen (in German) (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. pp. 372–3. ISBN 978-3-8047-1763-3.
{{cite book}}: CS1 maint: unrecognized language (link) - ↑ Wuttke W, Jarry H, Feleder C, Moguilevsky J, Leonhardt S, Seong JY, Kim K (1996). "The neurochemistry of the GnRH pulse generator". Acta Neurobiologiae Experimentalis. 56 (3): 707–13. PMID 8917899. Archived from the original on 2015-12-08.
- ↑ Jamil, George Leal (30 September 2013). Rethinking the Conceptual Base for New Practical Applications in Information Value and Quality. IGI Global. pp. 111–. ISBN 978-1-4666-4563-9. Archived from the original on 13 June 2021. Retrieved 27 August 2018.
- 1 2 Hara, Takuji (1 January 2003). Innovation in the Pharmaceutical Industry: The Process of Drug Discovery and Development. Edward Elgar Publishing. pp. 106–107. ISBN 978-1-84376-566-0. Archived from the original on 13 June 2021. Retrieved 27 August 2018.
- 1 2 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 730–. ISBN 978-1-4757-2085-3. Archived from the original on 13 June 2021. Retrieved 25 February 2018.
- 1 2 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 599–. ISBN 978-3-88763-075-1. Archived from the original on 2021-06-13. Retrieved 2018-02-25.
- 1 2 I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 164–. ISBN 978-94-011-4439-1. Archived from the original on 13 June 2021. Retrieved 25 February 2018.
- 1 2 "Leuprorelin". Archived from the original on 2018-02-25. Retrieved 2018-02-25.
- ↑ Sara K. Butler; Ramaswamy Govindan (25 October 2010). Essential Cancer Pharmacology: The Prescriber's Guide. Lippincott Williams & Wilkins. pp. 262–. ISBN 978-1-60913-704-5. Archived from the original on 13 June 2021. Retrieved 29 August 2018.
- ↑ Richard A. Lehne; Laura Rosenthal (25 June 2014). Pharmacology for Nursing Care - E-Book. Elsevier Health Sciences. pp. 1296–. ISBN 978-0-323-29354-9. Archived from the original on 11 August 2021. Retrieved 29 August 2018.
- ↑ Prostate Cancer. Demos Medical Publishing. 20 December 2011. pp. 503–. ISBN 978-1-935281-91-7. Archived from the original on 13 June 2021. Retrieved 29 August 2018.
- ↑ Geier M, Geier D (2005). "The potential importance of steroids in the treatment of autistic spectrum disorders and other disorders involving mercury toxicity". Med Hypotheses. 64 (5): 946–54. doi:10.1016/j.mehy.2004.11.018. PMID 15780490.
- ↑ Allen A (2007-05-28). "Thiomersal on trial: the theory that vaccines cause autism goes to court". Slate. Archived from the original on 2008-02-03. Retrieved 2008-01-30.
- ↑ "Testosterone regulation". Research Autism. 2007-05-07. Archived from the original on 2015-04-18. Retrieved 2015-04-09.
- 1 2 "Maryland medical board upholds autism doctor's suspension". Chicago Tribune. May 11, 2011. Archived from the original on October 21, 2011.
- ↑ "John and Jane Doe v. Ortho-Clinical Diagnostics, Inc Archived 2008-03-06 at the Wayback Machine", US District Court for the Middle District of North Carolina, July 6, 2006
- ↑ "Dr. Mark Geier Severely Criticized Archived 2016-12-02 at the Wayback Machine", Stephen Barrett, M.D., Casewatch.org
- ↑ Mills S, Jones T (2009-05-21). "Physician team's crusade shows cracks". Chicago Tribune. Archived from the original on 2009-05-25. Retrieved 2009-05-21.
- ↑ 'Miracle drug' called junk science: Powerful castration drug pushed for autistic children, but medical experts denounce unproven claims Archived 2013-12-03 at the Wayback Machine, Chicago Tribune, May 21, 2009
- ↑ Desmarchelier M, Lair S, Dunn M, Langlois I (2008). "Primary hyperaldosteronism in a domestic ferret with an adrenocortical adenoma". Journal of the American Veterinary Medical Association. 233 (8): 1297–301. doi:10.2460/javma.233.8.1297. PMID 19180717.
- ↑ Boari A, Papa V, Di Silverio F, Aste G, Olivero D, Rocconi F (2010). "Type 1 diabetes mellitus and hyperadrenocorticism in a ferret". Veterinary Research Communications. 34 (Suppl 1): S107–10. doi:10.1007/s11259-010-9369-2. PMID 20446034.
- ↑ Doraiswamy PM, Xiong GL (2006). "Pharmacological strategies for the prevention of Alzheimer's disease". Expert Opinion on Pharmacotherapy. 7 (1): 1–10. doi:10.1517/14656566.7.1.S1. PMID 16370917.
- 1 2 3 4 "Leuprorelin oral - Enteris BioPharma - AdisInsight". adisinsight.springer.com. Archived from the original on 4 November 2017. Retrieved 16 July 2018.
Further reading
- Shajnfeld, Adam; Krueger, Richard Bohn (July 2006). "Reforming (Purportedly) Non-Punitive Responses to Sexual Offending". Developments in Mental Health Law. 25: 81. SSRN 1077282.
External links
| External sites: |
|
|---|---|
| Identifiers: |
|