Osteosarcoma
| Osteosarcoma | |
|---|---|
| Other names: Osteogenic sarcoma (OGS) | |
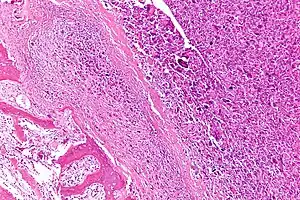 | |
| Intermediate-magnification micrograph of an osteosarcoma (center and right of image) adjacent to non-malignant bone (left-bottom of image): The top-right of the image has poorly differentiated tumor. Osteoid with a high density of malignant cells is seen between the non-malignant bone and poorly differentiated tumor (H&E stain). | |
| Specialty | Oncology |
| Symptoms | Bone pain[1] |
| Complications | Spread of the cancer, pathologic fracture[1][2] |
| Usual onset | 10 to 14 years old, > 65 years old[1] |
| Types | Primary, secondary[1] |
| Risk factors | Paget disease of bone, electrical burns, trauma, exposure to beryllium, alkylating agents, osteochondromatosis, enchondromatosis, fibrous dysplasia, prosthetics, bone infections[1] |
| Diagnostic method | Biopsy[2] |
| Differential diagnosis | Ewing sarcoma, metastasis to bone, giant cell tumor, fibrosarcoma, fibrous dysplasia, osteomyelitis[1] |
| Treatment | Surgery, chemotherapy, radiation therapy[1] |
| Prognosis | 5-year survival rate 76% for under 15 year old[2] |
| Frequency | 4.4 cases per million per year (in those under 25)[2] |
Osteosarcoma (OS) is a type of bone cancer of the osteogenic type.[1][3] The most common initial symptom is bone pain, which often gets worse with exercise.[1] In children more than half of cases occur in the long bones around the knee.[2] Spread of the cancer often occurs.[2] Other complications may include a pathologic fracture.[1]
The cause is often unclear.[1] Some cases occur secondary to Paget disease of bone, electrical burns, trauma, exposure to beryllium, alkylating agents, osteochondromatosis, enchondromatosis, fibrous dysplasia, prosthetics, or bone infections.[1] A number of genetic syndrome increase the risk including Li-Fraumeni syndrome, Bloom syndrome, or Werner syndrome.[1] It starts from primitive bone-forming mesenchymal cells.[1] Diagnosis is by biopsy.[2]
Treatment may include surgery, chemotherapy, and radiation therapy.[1] Ongoing follow up is required to look for re-occurrence.[1] The 5-year survival rate in those under 15 is about 76%.[2] Outcomes are worse in adults.[1]
About 4.4 cases per million per year occur in those under the age of 25.[2] It is most common in those 10 to 14 years old and those over the age of 65.[1] It represents about 5% of cancer in children and about 20% of tumors that start within bone.[1][2] The term "osteosarcoma" was introduced in 1805 by Alexis Boyer.[4]
Signs and symptoms

Many people first complain of pain that may be worse at night, may be intermittent and of varying intensity and may have been occurring for some time. Teenagers who are active in sports often complain of pain in the lower femur, or immediately below the knee. If the tumor is large, it can present as overt localised swelling. Sometimes a sudden fracture is the first symptom, because the affected bone is not as strong as normal bone and may fracture abnormally with minor trauma. In cases of more deep-seated tumors that are not as close to the skin, such as those originating in the pelvis, localised swelling may not be apparent.
Causes
Several research groups are investigating cancer stem cells and their potential to cause tumors along with genes and proteins causative in different phenotypes.[5][6][7] Radiotherapy for unrelated conditions may be a rare cause.[8]
- Familial cases where the deletion of chromosome 13q14 inactivates the retinoblastoma gene is associated with a high risk of osteosarcoma development.
- Bone dysplasias, including Paget's disease of bone, fibrous dysplasia, enchondromatosis, and hereditary multiple exostoses, increase the risk of osteosarcoma.
- Li–Fraumeni syndrome (germline TP53 mutation) is a predisposing factor for osteosarcoma development.
- Rothmund–Thomson syndrome (i.e. autosomal recessive association of congenital bone defects, hair and skin dysplasias, hypogonadism, and cataracts) is associated with increased risk of this disease.
- Large doses of Sr-90 emission from nuclear reactors, nicknamed bone seeker increases the risk of bone cancer and leukemia in animals, and is presumed to do so in people.[9]
There is no clear association between water fluoridation and cancer or deaths due to cancer, both for cancer in general and also specifically for bone cancer and osteosarcoma.[10] Series of research concluded that concentration of fluoride in water doesn't associate with osteosarcoma. The beliefs regarding association of fluoride exposure and osteosarcoma stem from a study of US National Toxicology program in 1990, which showed uncertain evidence of association of fluoride and osteosarcoma in male rats. But there is still no solid evidence of cancer-causing tendency of fluoride in mice.[11] Fluoridation of water has been practiced around the world to improve citizens' dental health. It is also deemed as major health success.[12] Fluoride concentration levels in water supplies are regulated, such as United States Environmental Protection Agency regulates fluoride levels to not be greater than 4 milligrams per liter.[13] Actually, water supplies already have natural occurring fluoride, but many communities chose to add more fluoride to the point that it can reduce tooth decay.[14] Fluoride is also known for its ability to cause new bone formation.[15] Yet, further research shows no osteosarcoma risks from fluoridated water in humans.[16] Most of the research involved counting the number of osteosarcoma cases in particular areas which has difference concentrations of fluoride in drinking water.[17] The statistic analysis of the data shows no significant difference in occurrences of osteosarcoma cases in different fluoridated regions.[18] Another important research involved collecting bone samples from people with osteosarcoma to measure fluoride concentration and compare them to bone samples of newly diagnosed malignant bone tumors. The result is that the median fluoride concentrations in bone samples of people with osteosarcoma and tumor controls are not significantly different.[19] Not only fluoride concentration in bones, fluoride exposures of people with osteosarcoma are also proven to be not different from healthy people.[20]
Mechanism
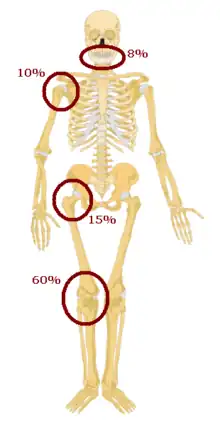
Osteosarcomas tend to occur at the sites of bone growth, presumably because proliferation makes osteoblastic cells in this region prone to acquire mutations that could lead to transformation of cells (the RB gene and p53 gene are commonly involved). Due to this tendency, high incidence of osteosarcoma is seen in some large dog breeds (St. Bernards and Great Danes). The tumor may be localized at the end of the long bone (commonly in the metaphysis). Most often it affects the proximal end of tibia or humerus, or distal end of femur. Osteosarcoma tends to affect regions around the knee in 60% of cases, 15% around the hip, 10% at the shoulder, and 8% in the jaw. The tumor is solid, hard, irregular ("fir-tree," "moth-eaten", or "sun-burst" appearance on X-ray examination) due to the tumor spicules of calcified bone radiating in right angles. These right angles form what is known as a Codman triangle, which is characteristic but not diagnostic of osteosarcoma. Surrounding tissues are infiltrated.
Microscopically: The characteristic feature of osteosarcoma is presence of osteoid (bone formation) within the tumor. Tumor cells are very pleomorphic (anaplastic), some are giant, numerous atypical mitoses. These cells produce osteoid describing irregular trabeculae (amorphous, eosinophilic/pink) with or without central calcification (hematoxylinophilic/blue, granular)—tumor bone. Tumor cells are included in the osteoid matrix. Depending on the features of the tumor cells present (whether they resemble bone cells, cartilage cells, or fibroblast cells), the tumor can be subclassified. Osteosarcomas may exhibit multinucleated osteoclast-like giant cells.[21]
Diagnosis

Family physicians and orthopedists rarely see a malignant bone tumor (most bone tumors are benign). The route to osteosarcoma diagnosis usually begins with an X-ray, continues with a combination of scans (CT scan, PET scan, bone scan, MRI) and ends with a surgical biopsy. A characteristic often seen in an X-ray is Codman's triangle, which is basically a subperiosteal lesion formed when the periosteum is raised due to the tumor. Films are suggestive, but bone biopsy is the only definitive method to determine whether a tumor is malignant or benign.
Most times, the early signs of osteosarcoma are caught on X-rays taken during routine dental check-ups. Osteosarcoma frequently develops in the mandible (lower jaw); accordingly, dentists are trained to look for signs that may suggest osteosarcoma. Even though radiographic findings for this cancer vary greatly, one usually sees a symmetrical widening of the periodontal ligament space. If the dentist has reason to suspects osteosarcoma or another underlying disorder, he or she would refer the person to an Oral & Maxillofacial surgeon for biopsy. A biopsy of suspected osteosarcoma outside of the facial region should be performed by a qualified orthopedic oncologist. The American Cancer Society states: "Probably in no other cancer is it as important to perform this procedure properly. An improperly performed biopsy may make it difficult to save the affected limb from amputation." It may also metastasise to the lungs, mainly appearing on the chest X-ray as solitary or multiple round nodules most common at the lower regions.
Osteosarcoma is the most common histological form of primary bone sarcoma.[22]
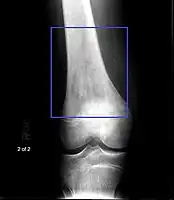 Osteosarcoma of the distal femur
Osteosarcoma of the distal femur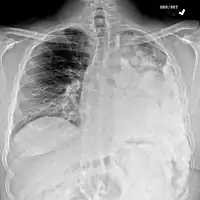 Metastatic osteosarcoma to the left chest
Metastatic osteosarcoma to the left chest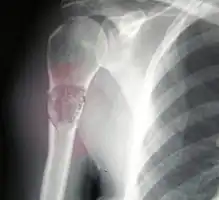 Telangiectactic osteosarcoma of the humerus
Telangiectactic osteosarcoma of the humerus
Variants
- Conventional: osteoblastic, chondroblastic, fibroblastic OS
- Telangiectatic OS
- Small cell OS
- Low-grade central OS
- Periosteal OS
- Paraosteal OS
- Secondary OS
- High-grade surface OS
- Extraskeletal OS
Treatment
A complete radical, surgical, en bloc resection of the cancer, is the treatment of choice in osteosarcoma.[22] Although about 90% of people are able to have limb-salvage surgery, complications, particularly infection, prosthetic loosening and non-union, or local tumor recurrence may cause the need for further surgery or amputation.
Mifamurtide is used after a person has had surgery to remove the tumor and together with chemotherapy to kill remaining cancer cells to reduce the risk of cancer recurrence. Also, the option to have rotationplasty after the tumor is taken out exists.[24]
People with osteosarcoma are best managed by a medical oncologist and an orthopedic oncologist experienced in managing sarcomas. Current standard treatment is to use neoadjuvant chemotherapy (chemotherapy given before surgery) followed by surgical resection. The percentage of tumor cell necrosis (cell death) seen in the tumor after surgery gives an idea of the prognosis and also lets the oncologist know if the chemotherapy regimen should be altered after surgery.
Standard therapy is a combination of limb-salvage orthopedic surgery when possible (or amputation in some cases) and a combination of high-dose methotrexate with leucovorin rescue, intra-arterial cisplatin, adriamycin, ifosfamide with mesna, BCD (bleomycin, cyclophosphamide, dactinomycin), etoposide, and muramyl tripeptide.[25] Rotationplasty may be used. Ifosfamide can be used as an adjuvant treatment if the necrosis rate is low.
Despite the success of chemotherapy for osteosarcoma, it has one of the lowest survival rates for pediatric cancer. The best reported 10-year survival rate is 92%; the protocol used is an aggressive intra-arterial regimen that individualizes therapy based on arteriographic response.[26] Three-year event-free survival ranges from 50% to 75%, and five-year survival ranges from 60% to 85+% in some studies. Overall, 65–70% people treated five years ago will be alive today.[27] These survival rates are overall averages and vary greatly depending on the individual necrosis rate.
Filgrastim or pegfilgrastim help with white blood cell counts and neutrophil counts. Blood transfusions and epoetin alfa help with anemia. Computational analysis on a panel of osteosarcoma cell lines identified new shared and specific therapeutic targets (proteomic and genetic) in osteosarcoma, while phenotypes showed an increased role of tumor microenvironments.[28]
Prognosis
Prognosis is separated into three groups.
- Stage I osteosarcoma is rare and includes parosteal osteosarcoma or low-grade central osteosarcoma. It has an excellent prognosis (>90%) with wide resection.
- Stage II prognosis depends on the site of the tumor (proximal tibia, femur, pelvis, etc.), size of the tumor mass, and the degree of necrosis from neoadjuvant chemotherapy. Other pathological factors such as the degree of p-glycoprotein, whether the tumor is cxcr4-positive,[29] or Her2-positive are also important, as these are associated with distant metastases to the lung. The prognosis of metastatic osteosarcoma improves with longer times to metastases (more than 12 months to 4 months), a smaller number of metastases, and their resectability. It is better to have fewer metastases than longer time to metastases. Those with a longer length of time (more than 24 months) and few nodules (two or fewer) have the best prognosis, with a two-year survival after the metastases of 50%, five-year of 40%, and 10-year of 20%. If metastases are both local and regional, the prognosis is worse.
- Stage III osteosarcoma with lung metastases depends on the resectability of the primary tumor and lung nodules, degree of necrosis of the primary tumor, and maybe the number of metastases. Overall survival prognosis is about 30%.[30]
Deaths due to malignant neoplasms of the bones and joints account for an unknown number of childhood cancer deaths. Mortality rates due to osteosarcoma have been declining at about 1.3% per year. Long-term survival probabilities for osteosarcoma have improved dramatically during the late 20th century and approximated 68% in 2009.[22]
Epidemiology
Osteosarcoma is the eighth-most common form of childhood cancer, comprising 2.4% of all malignancies in children, and about 20% of all primary bone cancers.[22]
Incidence rates for osteosarcoma in people under 20 years of age in the USA are estimated at 5.0 per million per year in the general population, with a slight variation between individuals of black, Hispanic, and white ethnicities (6.8, 6.5, and 4.6 per million per year, respectively). It is slightly more common in males (5.4 per million per year) than in females (4.0 per million per year).[22]
It originates more frequently in the metaphyseal region of tubular long bones, with 42% occurring in the femur, 19% in the tibia, and 10% in the humerus. About 8% of all cases occur in the skull and jaw, and another 8% in the pelvis.[22]
Around 300 of the 900 people diagnosed in the United States will die each year. A second peak in incidence occurs in the elderly, usually associated with an underlying bone pathology such as Paget's disease of bone.
Other animals

Presentation
The most commonly affected bones are the proximal humerus, the distal radius, the distal femur, and the tibia,[31] following the basic premise "far from the elbow, close to the knee". Other sites include the ribs, the mandible, the spine, and the pelvis. Rarely, osteosarcoma may arise from soft tissues (extraskeletal osteosarcoma). Metastasis of tumors involving the limb bones is very common, usually to the lungs. The tumor causes a great deal of pain, and can even lead to fracture of the affected bone. As with human osteosarcoma, bone biopsy is the definitive method to reach a final diagnosis. Osteosarcoma should be differentiated from other bone tumours and a range of other lesions, such as osteomyelitis. Differential diagnosis of the osteosarcoma of the skull in particular includes, among others, chondrosarcoma and the multilobular tumour of bone.[32][33]
Risk factors
Osteosarcoma is the most common bone tumor in dogs and typically afflicts middle-aged large and giant breed dogs such as Irish Wolfhounds, Greyhounds, German Shepherds, Rottweilers, mountain breeds (Great Pyrenees, St. Bernard, Leonberger, Newfoundland), Doberman Pinschers and Great Danes. It has a 10-fold greater incidence in dogs than humans.[34] A hereditary base has been shown in St. Bernard dogs.[35] Spayed/neutered dogs have twice the risk of intact ones to develop osteosarcoma.[36] Infestation with the parasite Spirocerca lupi can cause osteosarcoma of the esophagus.[37]
Treatment and prognosis
Amputation is the initial treatment, although this alone will not prevent metastasis. Chemotherapy combined with amputation improves the survival time, but most dogs still die within a year.[31] Surgical techniques designed to save the leg (limb-sparing procedures) do not improve the prognosis.
Some current studies indicate osteoclast inhibitors such as alendronate and pamidronate may have beneficial effects on the quality of life by reducing osteolysis, thus reducing the degree of pain, as well as the risk of pathological fractures.[38]
Cats
Osteosarcoma is also the most common bone tumor in cats, although not as frequently encountered, and most typically affects the rear legs. The cancer is generally less aggressive in cats than in dogs, so amputation alone can lead to a significant survival time in many affected cats, though post-amputation chemotherapy is recommended when a high grade is confirmed on histopathology.[31]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 Prater, S; McKeon, B (January 2020). "Osteosarcoma". PMID 31751058.
{{cite journal}}: Cite journal requires|journal=(help) - 1 2 3 4 5 6 7 8 9 10 "Osteosarcoma and Undifferentiated Pleomorphic Sarcoma of Bone Treatment (PDQ®)–Health Professional Version - National Cancer Institute". www.cancer.gov. 11 December 2020. Archived from the original on 23 July 2018. Retrieved 20 December 2020.
- ↑ WHO Classification of Tumours Editorial Board, ed. (2020). "Osteosarcoma". Soft Tissue and Bone Tumours: WHO Classification of Tumours. Vol. 3 (5th ed.). Lyon (France): International Agency for Research on Cancer. pp. 403–409. ISBN 978-92-832-4503-2. Archived from the original on 2021-06-13. Retrieved 2021-05-10.
- ↑ Jaffe, Norman; Bruland, Oyvind S.; Bielack, Stefan (2010). Pediatric and Adolescent Osteosarcoma. Springer Science & Business Media. p. 531. ISBN 978-1-4419-0284-9. Archived from the original on 2021-08-28. Retrieved 2020-12-20.
- ↑ Osuna D, de Alava E (2009). "Molecular pathology of sarcomas". Rev Recent Clin Trials. 4 (1): 12–26. doi:10.2174/157488709787047585. hdl:10261/61716. PMID 19149759. S2CID 15039305. Archived from the original on 2021-08-28. Retrieved 2019-11-15.
- ↑ Sharma, Ankush; Cinti, Caterina; Capobianco, Enrico (2017). "Multitype Network-Guided Target Controllability in Phenotypically Characterized Osteosarcoma: Role of Tumor Microenvironment". Frontiers in Immunology. 8: 918. doi:10.3389/fimmu.2017.00918. ISSN 1664-3224. PMC 5536125. PMID 28824643.
- ↑ Sharma, Ankush; Capobianco, Enrico (2017). "Immuno-Oncology Integrative Networks: Elucidating the Influences of Osteosarcoma Phenotypes". Cancer Informatics. 16: 117693511772169. doi:10.1177/1176935117721691. ISSN 1176-9351. PMC 5533255. PMID 28804242. Archived from the original on 2017-08-21. Retrieved 2017-08-21.
- ↑ Dhaliwal J, Sumathi VP, Grimer RJ (20 December 2009). "Radiation-induced periosteal osteosarcoma" (PDF). Grand Rounds. 10: 13–18. doi:10.1102/1470-5206.2010.0003 (inactive 2020-09-01). Archived (PDF) from the original on 2017-03-06. Retrieved 2017-03-06.
{{cite journal}}: CS1 maint: DOI inactive as of September 2020 (link) - ↑ "Sr-90 is known to increase the risk of bone cancer and leukemia in animals, and is presumed to do so in people; from google (nuclear reactor emit strontium) result 2". Archived from the original on 2017-07-20. Retrieved 2017-06-25.
- ↑ National Health and Medical Research Council (Australia). A systematic review of the efficacy and safety of fluoridation [PDF]. 2007 [Retrieved 2009-10-13]. ISBN 1-86496-415-4. Archived 2009-10-14 at the Wayback Machine "Archive copy" (PDF). Archived from the original on 2009-10-14. Retrieved 2020-04-27.
{{cite web}}: CS1 maint: archived copy as title (link) CS1 maint: bot: original URL status unknown (link) - ↑ "Water Fluoridation and Cancer Risk" Archived 2014-11-29 at the Wayback Machine, American Cancer Society, 6 June 2013.
- ↑ "Cancer myth: Fluoride and cancer" Archived 2014-09-14 at the Wayback Machine, Cancer Council Western Australia.
- ↑ "Basic Information about Fluoride in Drinking Water" Archived 2014-12-04 at the Wayback Machine, United States Environmental Protection Agency.
- ↑ "Community Water Fluoridation" Archived 2017-07-05 at the Wayback Machine, Centers of disease control and prevention.
- ↑ "Fluoride" Archived 2018-11-27 at the Wayback Machine, Australian government national health and medical research council.
- ↑ "Fluoridated Water" Archived 2014-12-02 at the Wayback Machine, National Cancer Institute.
- ↑ Blakey K, Feltbower RG, Parslow RC, James PW, Gómez Pozo B, Stiller C, Vincent TJ, Norman P, McKinney PA, Murphy MF, Craft AW, McNally RJ (14 January 2014). "Is fluoride a risk factor for bone cancer? Small area analysis of osteosarcoma and Ewing sarcoma diagnosed among 0-49-year-olds in Great Britain, 1980-2005". International Journal of Epidemiology. 43 (1): 224–234. doi:10.1093/ije/dyt259. PMC 3937980. PMID 24425828.
- ↑ Mahoney MC, Nasca PC, Burnett WS, Melius JM (April 1991). "Bone cancer incidence rates in New York State: time trends and fluoridated drinking water". American Journal of Public Health. 81 (4): 475–9. doi:10.2105/AJPH.81.4.475. PMC 1405037. PMID 2003628.
- ↑ Kim FM, Hayes C, Williams PL, Whitford GM, Joshipura KJ, Hoover RN, Douglass CW, National Osteosarcoma Etiology Group (October 2011). "An assessment of bone fluoride and osteosarcoma". Journal of Dental Research. 90 (10): 1171–6. doi:10.1177/0022034511418828. PMC 3173011. PMID 21799046.
- ↑ Gelberg KH, Fitzgerald EF, Hwang SA, Dubrow R (December 1995). "Fluoride exposure and childhood osteosarcoma: a case-control study". American Journal of Public Health. 85 (12): 1678–83. doi:10.2105/AJPH.85.12.1678. PMC 1615731. PMID 7503344.
- ↑ Papalas JA, Balmer NN, Wallace C, Sangüeza OP (June 2009). "Ossifying dermatofibroma with osteoclast-like giant cells: report of a case and literature review". Am J Dermatopathol. 31 (4): 379–83. doi:10.1097/DAD.0b013e3181966747. PMID 19461244.
- 1 2 3 4 5 6 Ottaviani G, Jaffe N (2009). The epidemiology of osteosarcoma. In: Jaffe N. et al. "Pediatric and Adolescent Osteosarcoma". Cancer Treatment and Research. Vol. 152. New York: Springer. pp. 3–13. doi:10.1007/978-1-4419-0284-9_1. ISBN 978-1-4419-0283-2. PMID 20213383.
- ↑ WHO
- ↑ Luke's Story: Surviving Osteosarcoma Archived 2014-05-18 at the Wayback Machine, Children's Cancer Research Fund. Accessed 2016-11-07.
- ↑ Sameer Rastogi, Aditi Aggarwal, Akash Tiwari et al. Chemotherapy in Nonmetastatic Osteosarcoma: Recent Advances and Implications for Developing Countries. https://ascopubs.org/doi/full/10.1200/JGO.2016.007336 Archived 2019-11-06 at the Wayback Machine
- ↑ Wilkins RM, Cullen JW, Odom L, Jamroz BA, Cullen PM, Fink K, Peck SD, Stevens SL, Kelly CM, Camozzi AB (June 2003). "Superior survival in treatment of primary nonmetastatic pediatric osteosarcoma of the extremity". Annals of Surgical Oncology. 10 (5): 498–507. doi:10.1245/ASO.2003.03.061. PMID 12794015. S2CID 32721347.
- ↑ Buecker PJ, Gebhardt M, Weber K (2005). "Osteosarcoma". ESUN. Archived from the original on 2009-04-21. Retrieved 2009-04-15.
- ↑ Sharma, Ankush; Cinti, Caterina; Capobianco, Enrico (2017). "Multitype Network-Guided Target Controllability in Phenotypically Characterized Osteosarcoma: Role of Tumor Microenvironment". Frontiers in Immunology. 8: 918. doi:10.3389/fimmu.2017.00918. ISSN 1664-3224. PMC 5536125. PMID 28824643.
- ↑ "osteosarcomasupport.org" (PDF). osteosarcomasupport.org. Archived (PDF) from the original on 2013-07-31. Retrieved 2012-11-13.
- ↑ Koshkina, NV; Corey, S (2008). "Novel Targets to Treat Osteosarcoma Lung Metastases". ESUN. Archived from the original on 2009-04-24. Retrieved 2009-04-14.
- 1 2 3 Morrison, Wallace B. (1998). Cancer in Dogs and Cats (1st ed.). Williams and Wilkins. ISBN 978-0-683-06105-5.
- ↑ Loukopoulos P, Thornton JR, Robinson WF (May 2003). "Clinical and pathologic relevance of p53 index in canine osseous tumors". Veterinary Pathology. 40 (3): 237–48. doi:10.1354/vp.40-3-237. PMID 12724563.
- ↑ Psychas, V; Loukopoulos, P; Polizopoulou, ZS; Sofianidis, G (March 2009). "Multilobular tumour of the caudal cranium causing severe cerebral and cerebellar compression in a dog". Journal of Veterinary Science. 10 (1): 81–3. doi:10.4142/jvs.2009.10.1.81. PMC 2801101. PMID 19255529.
- ↑ Withrow, S.J. (2003). "Limb Sparing Trials and Canine Osteosarcoma". Genes, Dogs and Cancer: 3rd Annual Canine Cancer Conference, 2003. Archived from the original on 2005-03-15. Retrieved 2006-06-16.
- ↑ Bech-Nielsen, S., Haskins, M. E., et al. (1978). "Frequency of osteosarcoma among first-degree relatives of St. Bernard dogs". J Natl Cancer Inst. 60 (2): 349–53. doi:10.1093/jnci/60.2.349. PMID 271748.
- ↑ Ru, B.; Terracini, G.; et al. (1998). "Host related risk factors for canine osteosarcoma". Vet J. 156 (1): 31–9. doi:10.1016/S1090-0233(98)80059-2. PMID 9691849.
- ↑ Ranen E, Lavy E, Aizenberg I, Perl S, Harrus S (2004). "Spirocercosis-associated esophageal sarcomas in dogs. A retrospective study of 17 cases (1997-2003)". Veterinary Parasitology. 119 (2–3): 209–221. doi:10.1016/j.vetpar.2003.10.023. PMID 14746980.
- ↑ Tomlin JL, Sturgeon C, Pead MJ, Muir P (29 July 2000). "Use of the bisphosphonate drug alendronate for palliative management of osteosarcoma in two dogs". The Veterinary Record. 147 (5): 129–32. doi:10.1136/vr.147.5.129. PMID 10958534. S2CID 35757270.
External links
- What is osteosarcoma? Archived 2009-04-21 at the Wayback Machine
| Classification | |
|---|---|
| External resources |
|