Tall Man lettering
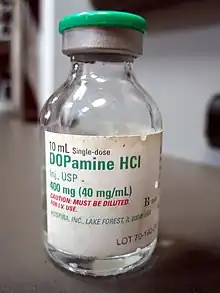
Tall man lettering (tall-man lettering or tallman lettering) is the practice of writing part of a drug's name in upper case letters to help distinguish sound-alike, look-alike drugs from one another in order to avoid medication errors.[1][2] For example, in tall man lettering, "prednisone" and "prednisolone" should be written "predniSONE" and "predniSOLONE", respectively. The Office of Generic Drugs of the US Food and Drug Administration (FDA) encourages manufacturers to use tall man lettering labels to visually differentiate their drugs' names,[1] and a number of hospitals, clinics, and health care systems use tall man lettering in their computerized order entry, automated dispensing machines, medication admission records, prescription labels, and drug product labels.[3]
Effectiveness
Wrong-drug errors have been found to occur at a rate of about one per thousand orders filled or dispensed.[4] Evidence regarding the effect of Tall Man lettering on error rates is mixed.
A 2004 eye-tracking study found that Tall Man lettering resulted in fewer errors when selecting a target drug from an array of choices.[5] Other laboratory-based studies of Tall Man lettering show a mixture of positive and null results, which may be further complicated due to demand characteristics of some of the studies.[4]
A 2016 time-series analysis of data from 42 children's hospitals over a 9-year period found no significant difference before and after a 2007 recommendation for hospitals to adopt Tall Man lettering.[6] However, this study has been criticized due to methodological limitations, such as not recording when and how Tall Man lettering was adopted, if at all, at each of the hospitals studied.[4]
Examples
The FDA and Institute for Safe Medication Practices (ISMP) published a list[7] of recommended Tall-Man Letters for look-alike drugs which includes, but is not limited to:
- acetaZOLAMIDE vs. acetoHEXAMIDE
- buPROPion vs. busPIRone
- chlorproMAZINE vs. chlorproPAMIDE
- clomiPHENE vs. clomiPRAMINE
- cycloSERINE vs. cycloSPORINE
- DAUNOrubicin vs. DOXOrubicin
- DOBUTamine vs. DOPamine
- hydrALAzine vs. hydrOXYzine
- TOLAZamide vs. TOLBUTamide
- vinBLAStine vs. vinCRIStine
See also
References
- 1 2 "Name Differentiation Project". FDA.gov. Center for Drug Evaluation and Research, U.S. Food and Drug Administration. Archived from the original on February 21, 2008. Retrieved January 10, 2022.
- ↑ "FAQ: 5. How should Tallman lettering be applied to look-alike/sound-alike drug name pairs?". ISMP.org. Institute for Safe Medication Practices. Archived from the original on December 18, 2007. Retrieved January 10, 2022.
- ↑ Examples: "University of Utah Health Care Pharmacy Alert #191" dated 2006/12/04, browsed 2007/12/20; Fraser Health 'Doctors in the Know' newsletter dated September, 2006, browsed 2007/12/20; Johns Hopkins Hospital "Pharmacy and Therapeutics Newsletter" dated July 2005, archived 2007/3/15.
- 1 2 3 Lambert, Bruce L; Schroeder, Scott R; Galanter, William L (2016). "Does Tall Man lettering prevent drug name confusion errors? Incomplete and conflicting evidence suggest need for definitive study". Editorial. BMJ Quality & Safety. 25 (4): 213–217. doi:10.1136/bmjqs-2015-004929. PMID 26700541.
- ↑ Filik, R; Purdy, K; Gale, A; Gerrett, D (December 2004). "Drug name confusion: Evaluating the effectiveness of capital ("Tall Man") letters using eye movement data". Social Science & Medicine. 59 (12): 2597–601. doi:10.1016/j.socscimed.2004.04.008. PMID 15474212.
- ↑ Zhong, Wenjun; Feinstein, James A; Patel, Neil S; Dai, Dingwei; Feudtner, Chirs (2016). "Tall Man lettering and potential prescription errors: A time series analysis of 42 children's hospitals in the USA over 9 years". BMJ Quality & Safety. 25 (4): 233–240. doi:10.1136/bmjqs-2015-004562.
- ↑ "Look-Alike Drug Names with Recommended Tall Man Letters" (PDF). ISMP.org. Institute for Safe Medication Practices. November 20, 2016. Retrieved 2022-01-10.
External links
- ISMP updates its list of drug name pairs with TALL man letters, November 18, 2010