Trifarotene
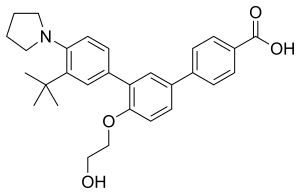 | |
| Names | |
|---|---|
| Trade names | Aklief |
| Other names | CD5789 |
IUPAC name
| |
| Clinical data | |
| Drug class | Retinoid[1] |
| Main uses | Acne[1] |
| Side effects | Itchiness, irritation, easy sunburns[1] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Topical |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a620004 |
| Legal | |
| License data |
|
| Legal status | |
| Chemical and physical data | |
| Formula | C29H33NO4 |
| Molar mass | 459.586 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Trifarotene, sold under the brand name Aklief, is a medication used for acne.[1] It is applied to the skin as a cream.[1] Moisturizer may also be used to help prevent irritation of the skin.[1]
Common side effects include itchiness, irritation, and easy sunburns.[1] There are concerns that use during pregnancy may harm the baby.[5] It is a retinoid; specifically a fourth generation selective retinoic acid receptor (RAR)-γ agonist.[1][6]
Trifarotene was approved for medical use in the United States, Canada, and Europe in 2019.[1][5] In the United States a 45 gram tube costs about 575 USD as of 2021.[7]
Medical uses
Trifarotene is used in the United States for the treatment of acne in people nine years of age and older.[4]
In both Canada and Australia, it is indicated for the treatment of acne of the face and/or the trunk in people twelve years of age and older.[2][3]
Dosage
It is used by applying it to the skin once per day at a dose of 0.005%.[1]
Side effects
Society and culture
Legal status
Trifarotene was approved for medical use in the United States in October 2019,[9] in Canada in November 2019,[3] and in Australia in January 2021.[2]
It was granted orphan drug designation for the treatment of congenital ichthyosis by both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).[10][11] In December 2019, its labelling and package leaflet text received a decentralised approval for 16 European countries.[12] The current approval status in each of these 16 countries is unknown.
References
- 1 2 3 4 5 6 7 8 9 10 "Trifarotene Monograph for Professionals". Drugs.com. Archived from the original on 4 March 2021. Retrieved 19 September 2021.
- 1 2 3 4 "Trifarotene Product Information". Therapeutic Goods Administration (TGA). Archived from the original on 23 May 2021. Retrieved 23 May 2021.
- 1 2 3 "Archive copy" (PDF). Archived (PDF) from the original on 23 May 2021. Retrieved 26 June 2021.
{{cite web}}: CS1 maint: archived copy as title (link) - 1 2 3 "Aklief- trifarotene cream". DailyMed. Archived from the original on 23 May 2021. Retrieved 22 May 2021.
- 1 2 "Australian Public Assessment Report for Trifarotene" (PDF). Archived (PDF) from the original on 23 September 2021. Retrieved 19 September 2021.
- ↑ Scott LJ (November 2019). "Trifarotene: First Approval". Drugs. 79 (17): 1905–1909. doi:10.1007/s40265-019-01218-6. PMID 31713811. S2CID 207964653. Archived from the original on 29 August 2021. Retrieved 26 June 2021.
- ↑ "Aklief Prices, Coupons & Savings Tips - GoodRx". GoodRx. Retrieved 19 September 2021.
- ↑ "Drug Trials Snapshots: Aklief". U.S. Food and Drug Administration (FDA). 11 October 2019. Archived from the original on 19 November 2019. Retrieved 18 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - ↑ "Drug Approval Package: Aklief". U.S. Food and Drug Administration (FDA). 21 October 2019. Archived from the original on 19 November 2019. Retrieved 18 November 2019.
- ↑ "Trifarotene Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 24 December 1999. Archived from the original on 29 August 2021. Retrieved 19 August 2020.
- ↑ "EU/3/20/2264". European Medicines Agency (EMA). 12 August 2020. Archived from the original on 9 January 2021. Retrieved 19 August 2020.
- ↑ "Galderma receives a positive outcome through the European Decentralised Procedure for AKLIEF (trifarotene 50 mcg/g cream), the first new retinoid molecule for acne in the European Union in 25 years". Businesswire. 20 December 2019. Archived from the original on 24 May 2021. Retrieved 26 June 2021.
External links
| Identifiers: |
|---|
- "Trifarotene". Drug Information Portal. U.S. National Library of Medicine (NLM). Archived from the original on 28 April 2021. Retrieved 26 June 2021.