Adapalene
 | |
 | |
| Names | |
|---|---|
| Trade names | Differin, Pimpal, Gallet, Adelene, others |
IUPAC name
| |
| Clinical data | |
| Drug class | Retinoids[1] |
| Main uses | Acne[2] |
| Side effects | Redness, skin peeling, dry skin, itchiness[2] |
| WHO AWaRe | UnlinkedWikibase error: ⧼unlinkedwikibase-error-statements-entity-not-set⧽ |
| Pregnancy category |
|
| Routes of use | Topical |
| External links | |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a604001 |
| Legal | |
| License data | |
| Legal status | |
| Pharmacokinetics | |
| Bioavailability | Very low |
| Excretion | Bile |
| Chemical and physical data | |
| Formula | C28H28O3 |
| Molar mass | 412.529 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Adapalene, sold under the brand name Differin among others, is a medication primarily used to treat acne.[2] Other uses may include molluscum contagiosum and actinic keratoses.[1] It is applied to the skin.[2]
Common side effects include redness, skin peeling, dry skin, and itchiness.[2] Other side effects may include sunburns.[2] It may result in less irritation than tazarotene.[1] It should not be used in pregnancy.[3] It is a third-generation retinoid and binds to the retinoic acid receptor (RAR).[1] It is more stable compared to tretinoin and tazarotene when exposed to sunlight.[1]
Adapalene was approved for medical use in the United States in 1996.[2] It is available as a generic medication.[4] In the United Kingdom 45 grams costs the NHS about £16 as of 2021.[3] This amount in the United States is about 45 USD.[4] It also comes mixed with benzoyl peroxide.[3]
Medical uses
Per the recommendations of the Global Alliance on Improving Outcomes of Acne, retinoids such as adapalene are considered first-line therapy in acne treatment and are to be used either independently or in conjunction with benzoyl peroxide and/or an antimicrobial agent, like clindamycin, for maximum efficacy.[5][6] Furthermore, adapalene, like other retinoids, increases the efficacy and penetration of other topical acne medications that are used in conjunction with topical retinoids as well as hastens the improvement of the post-inflammatory hyperpigmentation caused by acne.[5] In the long term, it can be used as maintenance therapy.[5] Adapalene is superior to tretinoin for the treatment of comedonal acne and is often used as a first-line agent.[7]
Off-label
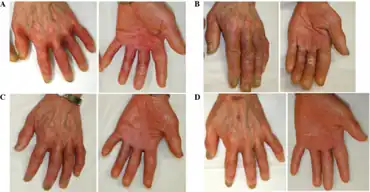
Adapalene has the unique ability to inhibit keratinocyte differentiation and decrease keratin deposition. This property makes adapalene an effective treatment for keratosis pilaris and callus. It may be used by men undergoing foreskin restoration to reduce excess keratin that forms a layer on the exterior of the human penis after circumcision. Other non-FDA approved indications that have been reported in the literature include treatment of warts, molluscum contagiosum, Darier disease, photoaging, pigmentary disorders, actinic keratoses and alopecia areata.[8]
Dosage
It is applied once a day.[3]
Side effects
Adapalene is known to cause mild adverse effects such as photosensitivity, irritation, redness, dryness, itching, and burning.[1] It is common (between 1% and 10% of users)[9] to experience a brief sensation of warmth or stinging, as well as dry skin, peeling and redness during the first 2–4 weeks of using the medication.[5][10] These effects are considered mild and generally decrease over time.[5][10] Any serious allergic reaction is rare.[10] Furthermore, of the three topical retinoids, adapalene is often regarded as the most tolerable.[8]
Pregnancy
Use of topical adapalene in pregnancy has not been well studied, but has a theoretical risk of retinoid embryopathy.[11] Thus far, there is no evidence that the cream causes problems in the baby if used during pregnancy. Use is at the consumer's own risk.[12]
According to the Drugs and Lactation Database, topical adapalene has poor systemic absorption and results in low blood levels (less than 0.025 mcg/L) despite long term use, suggesting that there is low risk of harm for a nursing infant.[13] However, it is recommended that the topical medication should not be applied to the nipple or any other area that may come into direct contact with the infant's skin.[13]
Interactions
Adapalene has been shown to enhance the efficacy of topical clindamycin, although adverse effects are also increased.[14][15] Application of adapalene gel to the skin 3–5 minutes before application of clindamycin enhances penetration of clindamycin into the skin, which may enhance the overall efficacy of the treatment as compared to clindamycin alone.[16]
Pharmacology
Unlike the retinoid tretinoin (Retin-A), adapalene has also been shown to retain its efficacy when applied at the same time as benzoyl peroxide due to its more stable chemical structure.[17] Furthermore, photodegradation of the molecule is less of a concern in comparison to tretinoin and tazarotene.[8]
Pharmacokinetics
Absorption of adapalene through the skin is low. A study with six acne patients treated once daily for five days with two grams of adapalene cream applied to 1,000 cm2 (160 sq in) of skin found no quantifiable amounts, or less than 0.35 ng/mL of the drug, in the patients' blood plasma.[18] Controlled trials of chronic users of adapalene have found drug levels in the patients' plasma to be 0.25 ng/mL.[11]
Pharmacodynamics
Adapalene is highly lipophilic. When applied topically, it readily penetrates hair follicles and absorption occurs 5 minutes after topical application.[1] After penetration into the follicle, adapalene binds to nuclear retinoic acid receptors (namely retinoic acid receptor beta and gamma).[6][11] These complexes then bind to the retinoid X receptor which induces gene transcription by binding to specific DNA sites, thus modulating downstream keratinocyte proliferation and differentiation.[1][11] This results in normalization of keratinocyte differentiation, allowing for decreased microcomedone formation, decreased clogging of pores, and increased exfoliation by increasing cell turnover.[8][11][19] Adapalene is also regarded as an anti-inflammatory agent, as it suppresses the inflammatory response stimulated by the presence of Cutibacterium acnes,[8] and inhibits both lipoxygenase activity and the oxidative metabolism of arachidonic acid into prostaglandins.[11]
Adapalene selectively targets retinoic acid receptor beta and retinoic acid receptor gamma when applied to epithelial cells such as those found in the skin.[20] Its agonism of the gamma subtype is largely responsible for adapalene's observed effects. In fact, when adapalene is applied in conjunction with a retinoic acid receptor gamma antagonist, adapalene loses clinical efficacy.[21]
Retinization is a common temporary phenomenon reported by patients when initiating use of retinols.[22] Within the initial period of treatment, skin can become red, irritated, dry and may burn or itch from retinol application; however, this tends to resolve within four weeks with once a day use.[22]
History
Adapalene is a research product of Galderma Laboratories, France.[23] Adapalene was approved in 1996 by the U.S. Food and Drug Administration (FDA) for use in the treatment of acne.[24]
Research
A study has concluded that adapalene can be used to treat plantar warts and may help clear lesions faster than cryotherapy.[25]
References
- 1 2 3 4 5 6 7 8 Tolaymat, L; Zito, PM (January 2021). "Adapalene". PMID 29494115.
{{cite journal}}: Cite journal requires|journal=(help) - 1 2 3 4 5 6 7 "Adapalene Monograph for Professionals". Drugs.com. Archived from the original on 31 January 2021. Retrieved 13 January 2022.
- 1 2 3 4 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1313. ISBN 978-0857114105.
- 1 2 "Adapalene Prices, Coupons & Savings Tips - GoodRx". GoodRx. Archived from the original on 27 June 2020. Retrieved 13 January 2022.
- 1 2 3 4 5 Kolli, Sree S.; Pecone, Danielle; Pona, Adrian; Cline, Abigail; Feldman, Steven R. (2019-01-23). "Topical Retinoids in Acne Vulgaris: A Systematic Review". American Journal of Clinical Dermatology. 20 (3): 345–365. doi:10.1007/s40257-019-00423-z. ISSN 1179-1888. PMID 30674002. S2CID 59225325.
- 1 2 Xiang, Leihong Flora; Troielli, Patricia; Lozada, Vicente Torres; Tan, Jerry; Suh, Dae Hun; See, Jo-Ann; Piquero-Martin, Jaime; Perez, Montserrat; Orozco, Beatriz (2018-02-01). "Practical management of acne for clinicians: An international consensus from the Global Alliance to Improve Outcomes in Acne". Journal of the American Academy of Dermatology. 78 (2): S1–S23.e1. doi:10.1016/j.jaad.2017.09.078. hdl:10067/1492720151162165141. ISSN 0190-9622. PMID 29127053. S2CID 31654121. Archived from the original on 2021-11-04. Retrieved 2021-09-12.
- ↑ Asai, Yuka; Baibergenova, Akerke; Dutil, Maha; Humphrey, Shannon; Hull, Peter; Lynde, Charles; Poulin, Yves; Shear, Neil H.; Tan, Jerry; Toole, John; Zip, Catherine (2 February 2016). "Management of acne: Canadian clinical practice guideline". Canadian Medical Association Journal. 188 (2): 118–126. doi:10.1503/cmaj.140665. PMC 4732962. PMID 26573753.
- 1 2 3 4 5 Tolaymat, Leila; Zito, Patrick M. (2018), "Adapalene", StatPearls, StatPearls Publishing, PMID 29494115, archived from the original on 2020-11-11, retrieved 2019-03-13
- ↑ "Differin". Swedish Drug Formulary. Archived from the original on 2017-12-11. Retrieved 2017-12-11.
- 1 2 3 "Adapalene Gel". WebMD. Archived from the original on 2017-12-11. Retrieved 2017-12-11.
- 1 2 3 4 5 6 Piskin, Suleyman; Uzunali, Erol (August 2007). "A review of the use of adapalene for the treatment of acne vulgaris". Therapeutics and Clinical Risk Management. 3 (4): 621–624. ISSN 1176-6336. PMC 2374937. PMID 18472984.
- ↑ "FDA approves Differin Gel 0.1% for over-the-counter use to treat acne". July 8, 2016. Archived from the original on 11 July 2016. Retrieved 14 July 2016.
- 1 2 "Adapalene", Drugs and Lactation Database (LactMed), National Library of Medicine (US), 2006, PMID 30000483, archived from the original on 2021-11-04, retrieved 2019-03-13
- ↑ Wolf JE, Kaplan D, Kraus SJ, Loven KH, Rist T, Swinyer LJ, Baker MD, Liu YS, Czernielewski J (September 2003). "Efficacy and tolerability of combined topical treatment of acne vulgaris with adapalene and clindamycin: a multicenter, randomized, investigator-blinded study". Journal of the American Academy of Dermatology. 49 (3 Suppl): S211-7. doi:10.1067/S0190-9622(03)01152-6. PMID 12963897.
- ↑ Jain, GauravK; Ahmed, FarhanJ (2007). "Adapalene pretreatment increases follicular penetration of clindamycin: In vitro and in vivo studies". Indian Journal of Dermatology, Venereology and Leprology. 73 (5): 326–9. doi:10.4103/0378-6323.34010. ISSN 0378-6323. PMID 17921613.
- ↑ Jain GK, Ahmed FJ (2007). "Adapalene pretreatment increases follicular penetration of clindamycin: in vitro and in vivo studies" (PDF). Indian Journal of Dermatology, Venereology and Leprology. 73 (5): 326–9. doi:10.4103/0378-6323.34010. PMID 17921613. Archived (PDF) from the original on 2021-11-04. Retrieved 2021-09-12.
- ↑ Martin B, Meunier C, Montels D, Watts O (October 1998). "Chemical stability of adapalene and tretinoin when combined with benzoyl peroxide in presence and in absence of visible light and ultraviolet radiation". The British Journal of Dermatology. 139 Suppl 52: 8–11. doi:10.1046/j.1365-2133.1998.1390s2008.x. PMID 9990414. S2CID 43287596.
- ↑ "DIFFERIN® (adapalene) Cream, 0.1% Label" (PDF). FDA. May 25, 2000. Archived (PDF) from the original on 16 February 2017. Retrieved 4 Oct 2011.
- ↑ "DIFFERIN® (adapalene) Gel, 0.3%" (PDF). Archived (PDF) from the original on April 7, 2021. Retrieved March 12, 2019.
- ↑ Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G (2006). "Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety". Clinical Interventions in Aging. 1 (4): 327–48. doi:10.2147/ciia.2006.1.4.327. PMC 2699641. PMID 18046911.
- ↑ Michel S, Jomard A, Démarchez M (October 1998). "Pharmacology of adapalene". The British Journal of Dermatology. 139 Suppl 52: 3–7. doi:10.1046/j.1365-2133.1998.1390s2003.x. PMID 9990413. S2CID 23084886.
- 1 2 "Differin Gel: An Over-the-Counter Retinoid for Acne". www.differin.com. Archived from the original on 2019-03-25. Retrieved 2019-03-25.
- ↑ US Patent 4717720A, Shroot B, Eustache J, Bernardon J-M, "Benzonaphthalene derivatives and compositions", published 1988-01-05, issued 1988-01-05, assigned to Galderma Research and Development SNC
- ↑ "FDA approval of DIFFERIN® (adapalene) Solution, 0.1%". FDA. May 31, 1996. Archived from the original on 28 June 2017. Retrieved 29 May 2017.
- ↑ Gupta, Ramji; Gupta, Sarthak (2015). "Topical Adapalene in the Treatment of Plantar Warts; Randomized Comparative Open Trial in Comparison with Cryo-Therapy". Indian Journal of Dermatology. 60 (1): 102. doi:10.4103/0019-5154.147835. ISSN 0019-5154. PMC 4318023. PMID 25657417.
External links
| External sites: |
|
|---|---|
| Identifiers: |