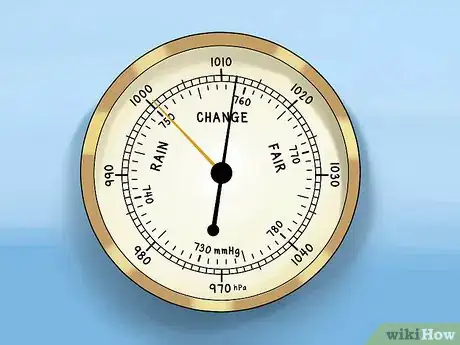This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
This article has been viewed 218,155 times.
You can use the barometric pressure to predict or analyze the weather. In a real-world situation, you'll use a barometer to measure the pressure, and then you will convert the reading to units that are more convenient for you to use.
Steps
Reading a Barometer
-
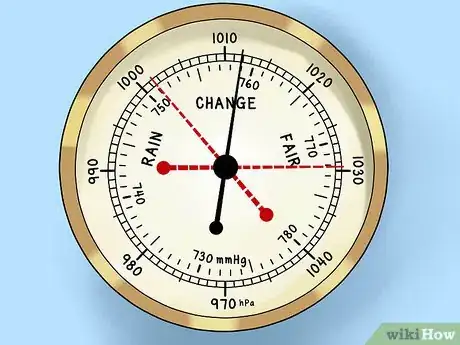
1Look for the trend. In gauging weather trends and analysis, the absolute value of the pressure is nowhere nearly as significant as its "trend." Namely: is the barometric pressure rising, is it falling, or is it holding steady? Watch the barometer needle and record its movements.[1]
- The dials of older barometers often feature artistically-drawn backgrounds to indicate weather conditions like storms, strong winds, and clear skies. For all their aesthetic, these backgrounds can be misleading. The movement of the barometer needle has far more bearing upon the coming weather.
- If you have an old, traditional mercury tube-type barometer, then you may need to watch the meniscus: the highest curve of the liquid mercury that fills the cylinder.
-
2Look at the reading. To determine the trend in a barometer, you need to compare the current pressure reading to a past pressure reading. Calculate the difference in pressure between the current reading and the reading from an hour ago.[2]
- In many barometers, you can manually set a needle to mark a point on the pressure gauge. The needle will remain at this point to help you gauge recent trends in the pressure.
Advertisement -
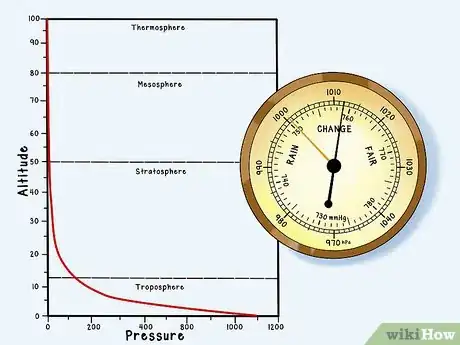
3Know that atmospheric pressure decreases more or less exponentially with altitude. The higher you go, the lower the pressure. This means that a barometric pressure that would send someone straight through the trapdoors of a storm cellar at sea level along the coast of Costa Rica would be perfectly commonplace in the midst of summer in the mile-high city of Denver.[3]
Calculating Pressure
-
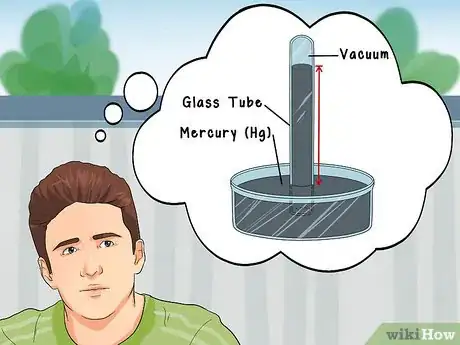
1Understand the origins of barometric measurement. The Italian physicist Torricelli conceived the first barometer from the fact that the average pressure of the atmosphere is capable of "sucking" 76 centimeters (760 mm) of mercury (Hg, which is a liquid metal at STP) up the inside bore of an evacuated glass tube. Later mathematicians have come up with other units of pressure, but the traditional unit remains mm Hg: millimeters of mercury.[4]
-
2Know the units of pressure. Pressure is a measure of force per unit area, and there are various ways to describe both the force and the area.[5] Atmospheric pressure is usually expressed in psi (pounds per square inch). It can also be expressed in "atmospheres" – one atmosphere is 14.7 psi. In the U.S., it is common to speak of air pressure in "inches of mercury," as one might read the pressure from a barometer. In meteorology, air pressure is most often expressed in "millibars:" each millibar is exactly one dyne (gm-cm/sec^2) per square centimeter in the c.g.s. system of units.[6]
- 14.7 psi is a rough average of barometric pressure at sea level and at STP (standard temperature and pressure.) STP is an internationally-accepted "general state" of the atmosphere. The 14.7 figure has been averaged over a large number of measurements, all either taken at sea level or corrected to sea level. Atmospheres are rarely used in meteorology.[7]
- The millibar is an especially convenient unit of pressure for atmospheric studies. 1033 millibars is the pressure equivalent to one atmosphere, 14.7 psi, or 30 inches of mercury. Most weather maps and all aeronautical weather charts are in millibars, and the pressure at sea level is usually very close to 1000 millibars.
- Nearly all barometers in the U.S. are graduated in units of inches of mercury. The barometer is read to the nearest hundredth of an inch, such as "29.93 inches." Similarly, aircraft altimeter settings are universally given by control towers in inches of mercury corrected to sea level, regardless of the altitude of the airfield.
-
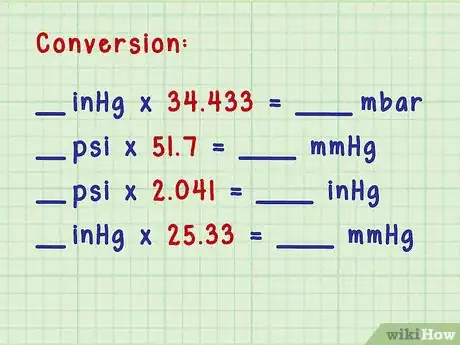
3Convert between units of pressure. If you’re simply converting a measurement from one unit of pressure to another, you can learn the multipliers to convert between millibars, psi, atmospheres, and mm of mercury.[8]
- Convert from inches of mercury (read from the barometer) to millibars: if you know the inches of mercury, simply multiply by 34.433.
- Convert from psi to mm of mercury: multiply the psi by 51.7.
- Convert from psi to inches of mercury: multiply the psi measurement by 2.041.
- Convert from inches of Hg (mercury) to mm Hg: multiply the measurement in inches by 25.33.
Community Q&A
-
QuestionHow can I solve this problem? 103 kPa = how many inches mercury?
 KickbuttowskiCommunity Answer1 kpa= 0.295 inches of mercury.Then multiplying it by 103 ,I.e 1 kpa ×103 = 0.295 ×103 inches=30.4159 inches. Therefore, 103 kpa =30.4159 inches of mercury.
KickbuttowskiCommunity Answer1 kpa= 0.295 inches of mercury.Then multiplying it by 103 ,I.e 1 kpa ×103 = 0.295 ×103 inches=30.4159 inches. Therefore, 103 kpa =30.4159 inches of mercury. -
QuestionWould 962 be pleasant or gloomy?
 Community AnswerUsually anything less than 1000 points to gloomy weather. The lower you are from 1000, the gloomier the weather.
Community AnswerUsually anything less than 1000 points to gloomy weather. The lower you are from 1000, the gloomier the weather. -
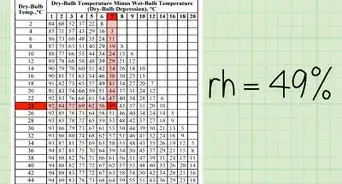
QuestionAfter I read the dry bulb and wet bulb temperature from the given hydrometer, how do I find out the relative humidity of air from the given relative humidity and table?
 Community AnswerTry to find the difference between the wet-bulb and dry-bulb temperatures. After that, take the dry-bulb temperature and the difference you calculated, and where the columns meet on the chart is your answer. You can also do that to find the dew-point temperature if you have the dew-point chart.
Community AnswerTry to find the difference between the wet-bulb and dry-bulb temperatures. After that, take the dry-bulb temperature and the difference you calculated, and where the columns meet on the chart is your answer. You can also do that to find the dew-point temperature if you have the dew-point chart.
References
- ↑ https://www.artofmanliness.com/lifestyle/gear/fair-or-foul-how-to-use-a-barometer/
- ↑ https://www.artofmanliness.com/lifestyle/gear/fair-or-foul-how-to-use-a-barometer/
- ↑ https://www.artofmanliness.com/lifestyle/gear/fair-or-foul-how-to-use-a-barometer/
- ↑ http://chemed.chem.purdue.edu/genchem/history/torricelli.html
- ↑ http://www.sparknotes.com/chemistry/gases/pressure/section1.rhtml
- ↑ https://www.unitconverters.net/pressure/millibar-to-dyne-square-centimeter.htm
- ↑ https://ntrs.nasa.gov/citations/19770009539
- ↑ http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/barfor.html
About This Article
To calculate barometric pressure, look at a barometer and write down the pressure reading. Then, check back in an hour and write down the new reading. Once you have both readings, subtract the current pressure from the pressure an hour ago to determine how much the barometric pressure has risen or fallen. To learn how to convert between units of barometric pressure, scroll down!