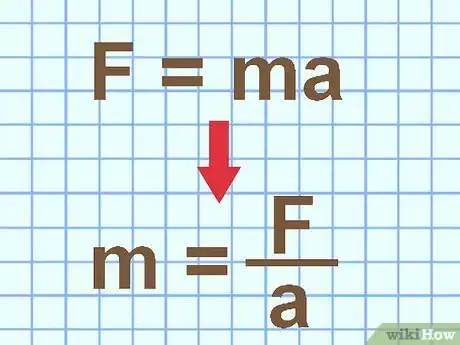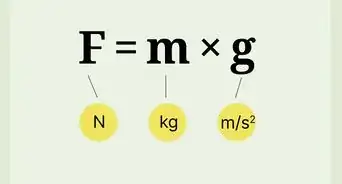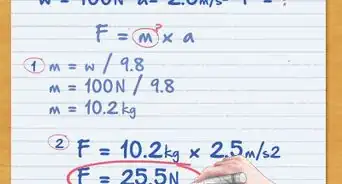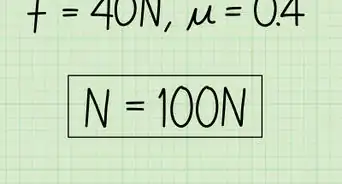This article was co-authored by Sean Alexander, MS. Sean Alexander is an Academic Tutor specializing in teaching mathematics and physics. Sean is the Owner of Alexander Tutoring, an academic tutoring business that provides personalized studying sessions focused on mathematics and physics. With over 15 years of experience, Sean has worked as a physics and math instructor and tutor for Stanford University, San Francisco State University, and Stanbridge Academy. He holds a BS in Physics from the University of California, Santa Barbara and an MS in Theoretical Physics from San Francisco State University.
There are 7 references cited in this article, which can be found at the bottom of the page.
This article has been viewed 1,083,162 times.
Mass is one of the fundamental properties of an object in Physics, and is a measurement of how much matter there is in something. Matter is any substance that you can touch — anything that takes up physical space and has volume. Often, mass is related to size, but this isn’t a perfect relationship, as objects like a large hot-air balloon often have less mass than a small boulder. To calculate mass, you’ll first need the density and volume of the object. Read on for details of the formula and to learn about different types of mass across scientific disciplines.
Steps
Finding Mass from Density and Volume
-
1Look up the object's density. Density measures how tightly the matter in an object is packed together. Each material has its own density, which you can look up online or in a textbook. The scientific unit of density is kilograms per cubic meter (kg/m3), but you can use grams per cubic centimeter (g/cm3) for smaller objects.
- Use this formula to convert between these units: 1,000 kg/m3 = 1 g/cm3
- The density of liquids is often measured in kilograms per liter (kg/L) or grams per milliliter (g/mL) instead. These units are equivalent: 1 kg/L = 1 g/mL.
- Example: Diamond has a density of 3.52 g/cm3.
-
2Measure the object's volume. The volume is the amount of space the object occupies. Measure the volume of solids in cubic meters (m3) or cubic centimeters (cm3), and the volume of liquids in liters (L) or milliliters (mL). The formula for volume depends on the shape of the object. Refer to this article for common shapes.
- Use the same unit that appears as part of your density measurement.
- Example: Since we measured the density of diamond in g/cm3, we should measure our diamond's volume in cm3. Let's say our diamond's volume is 5,000 cm3.
Advertisement -
3Multiply the volume and density together. Multiply your two numbers together, and you'll know the mass of your object.[1] Keep track of the units as you do this, and you'll see that you end up with units of mass (kilograms or grams).
- Example: We have a diamond with volume 5,000 cm3 and density 3.52 g/cm3. To find the diamond's mass, multiply 5,000 cm3 x 3.52 g/cm3 = 17,600 grams.
Solving for Mass in Other Science Problems
-
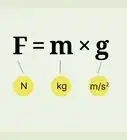
1Determine mass with force and acceleration. Newton's second law of motion states that force equals mass times acceleration: F = ma. If you know the net force on the object, and it's acceleration, you can rearrange this formula to find the mass: m = F / a.
- Force is measured in N (newton), which you can also write as (kg * m)/ s2. Acceleration is measured in m/s2. When you calculate F / a, the units cancel to give you an answer in kilograms (kg).[2]
-
2Understand mass and weight. Mass is the amount of matter in an object; this does not change unless you cut off part of the object, or attach more material. Weight is a measurement of gravity's effect on mass. If you move the object to an area with different gravity (such as from the earth to the moon), it's weight will change, but it's mass will not. [3]
- An object with more mass does weigh more than an object with less mass, if they're experiencing the same gravity.
-
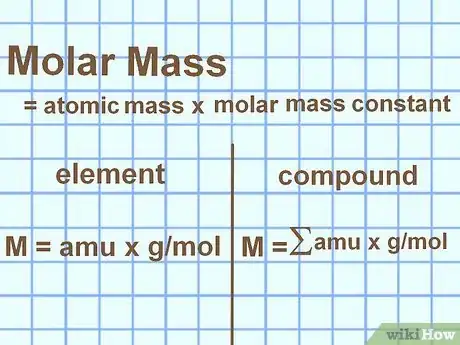
3Calculate molar mass. If you're doing your chemistry homework, you may come across the term "molar mass." This is a related concept, but instead of measuring an object, you measure exactly one mole of a substance. Here's how to calculate it in most contexts:
- For an element: look up the atomic mass of the element or compound you are measuring. This will be in "atomic mass units" (amu). Multiply by the molar mass constant, 1 g/mol, to put it into standard molar mass units: g/mol.
- For a compound: add the atomic masses of each atom in the compound to find the total amu of the molecule. Multiply this total by 1 g/mol.
Measuring Mass with a Balance
-
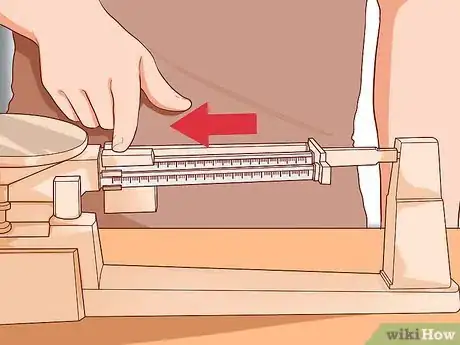
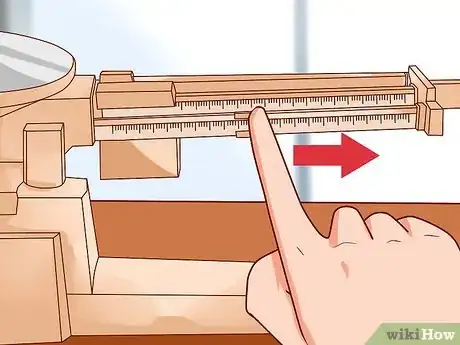
1Use a triple-beam balance. The balance is a device widely used to calculate an object's mass. The balance has three beams. These beams carry weights. [4] The weights allow you to move known masses along the beams.[5]
- The triple beam balance is not affected by gravity. Thus, it gives a true measurement of mass. It works by comparing a known mass to an unknown mass.
- The middle beam reads in 100g increments. The far beam reads in 10g increments. The weights will sit in a notch. The weight on the front beam can read from 0 to 10 grams.
- You should be able to get a very precise measurement of mass with this balance. The reading error for a triple-beam balance is only 0.06 grams. Think of the triple-beam balance as operating like a teeter-totter.[6]
-
2Move the three sliders to their leftmost positions. You want to do this maneuver when the pan is empty. You want the balance to read zero.
- If the indicator on the far right does not align with the fixed mark, you should calibrate the balance by turning the set screw that you will find on the left under the pan.
- The reason you need to do this is because you need to make sure that the empty pan is 0.000g so its weight does not skew the mass reading you ultimately get. The weight of the container or pan is called its tare.
- You can also set the pan to 0 by screwing the knob under the pan in or out. Again, the balance must read zero. Place the object to be measured on the pan. You are now ready to determine the object’s mass using the sliding beams.
-
3Move the sliding beams one at a time. First, move the 100-gram slider along the beam to the right first. Do this until the indicator drops below the fixed mark. The position that is to the left of this point indicates the number of hundreds of grams. You are sliding it one notch at a time.
- Move the 10-gram slider along the beam to the right. Do this until the indicator drops below the fixed mark. The notched position immediately to the left of this point indicates the number of tens of grams.
- The beam in the front does not have notches. You can move the slider anywhere you want on the beam. The beam’s boldface numbers are grams. The tick marks between the boldface numbers indicate tenths of grams.
-
4Calculate the mass. You are now ready to find the mass of the object you placed in the pan. To do so, you should add the numbers from the three beams.
- Read the front scale as you would a ruler. You can read it to the nearest half tick mark.
- For example, let's say you are trying to measure a can of soda. If the rear weight is in the notch that reads 70g, if the middle weight is in the notch reading 300g, and if the the front beam weight is 3.34g, then the can of soda weighs 373.34g.
Calculator, Practice Problems, and Answers
Community Q&A
-
QuestionHow do you calculate the mass of a solution?
 wikiHow Staff EditorThis answer was written by one of our trained team of researchers who validated it for accuracy and comprehensiveness.
wikiHow Staff EditorThis answer was written by one of our trained team of researchers who validated it for accuracy and comprehensiveness.
Staff Answer wikiHow Staff EditorStaff AnswerTo find the total mass of a solution, you’ll need to add the mass of the solute to the mass of the solvent. If you don’t know the mass of the solvent or the solute, you can calculate them if you know their density and volume.
wikiHow Staff EditorStaff AnswerTo find the total mass of a solution, you’ll need to add the mass of the solute to the mass of the solvent. If you don’t know the mass of the solvent or the solute, you can calculate them if you know their density and volume. -
QuestionHow do you calculate mass from weight?
 wikiHow Staff EditorThis answer was written by one of our trained team of researchers who validated it for accuracy and comprehensiveness.
wikiHow Staff EditorThis answer was written by one of our trained team of researchers who validated it for accuracy and comprehensiveness.
Staff Answer wikiHow Staff EditorStaff AnswerDivide the object’s weight by the acceleration of gravity to find the mass. You’ll need to convert the weight units to Newtons. For example, 1 kg = 9.807 N. If you’re measuring the mass of an object on Earth, divide the weight in Newtons by the acceleration of gravity on Earth (9.8 meters/second2) to get mass.
wikiHow Staff EditorStaff AnswerDivide the object’s weight by the acceleration of gravity to find the mass. You’ll need to convert the weight units to Newtons. For example, 1 kg = 9.807 N. If you’re measuring the mass of an object on Earth, divide the weight in Newtons by the acceleration of gravity on Earth (9.8 meters/second2) to get mass. -
QuestionHow do you calculate mass from weight?
 wikiHow Staff EditorThis answer was written by one of our trained team of researchers who validated it for accuracy and comprehensiveness.
wikiHow Staff EditorThis answer was written by one of our trained team of researchers who validated it for accuracy and comprehensiveness.
Staff Answer wikiHow Staff EditorStaff AnswerDivide the object’s weight by the acceleration of gravity to find the mass. You’ll need to convert the weight units to Newtons. For example, 1 kg = 9.807 N. If you’re measuring the mass of an object on Earth, divide the weight in Newtons by the acceleration of gravity on Earth (9.8 meters/second2) to get mass.
wikiHow Staff EditorStaff AnswerDivide the object’s weight by the acceleration of gravity to find the mass. You’ll need to convert the weight units to Newtons. For example, 1 kg = 9.807 N. If you’re measuring the mass of an object on Earth, divide the weight in Newtons by the acceleration of gravity on Earth (9.8 meters/second2) to get mass.
Warnings
- Don't use pounds and ounces to measure mass; these are units of weight, and not used in scientific contexts. Technically, in the United States, the measurement of mass is called a “slug.”[9]⧼thumbs_response⧽
References
- ↑ http://socratic.org/questions/how-do-you-calculate-mass-using-density-and-volume
- ↑ https://www.easycalculation.com/physics/classical-physics/force.php
- ↑ http://www.engineeringtoolbox.com/mass-weight-d_589.html
- ↑ https://study.com/learn/lesson/triple-beam-balance.html
- ↑ https://study.com/learn/lesson/triple-beam-balance.html
- ↑ https://study.com/learn/lesson/triple-beam-balance.html
- ↑ http://www.calculatorsoup.com/calculators/physics/density.php
- ↑ https://www.calculator.net/mass-calculator.html
- ↑ https://units.fandom.com/wiki/Slug#cite_note-Shigley-1
About This Article
To calculate the mass of an object, look up the recorded density of the object online or in a textbook, which will be in units of kg/m3 or g/cm3. Then, multiply the density of the object by it’s measured volume. Make sure that your measurements for volume and density are in the same units! For example, if you have a diamond with a volume of 5,000 cm3 and density of 3.52 g/cm3, multiply 5,000 cm3 by 3.52 g/cm3 to get the mass of 17,600 grams. If you want to learn how to find mass using a balance scale, keep reading the article!