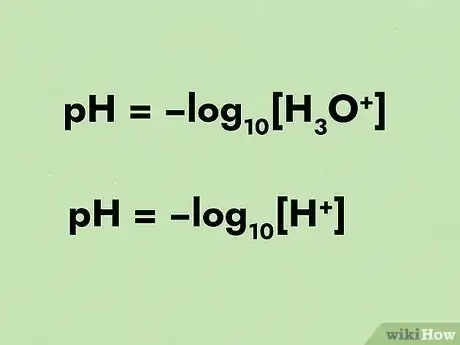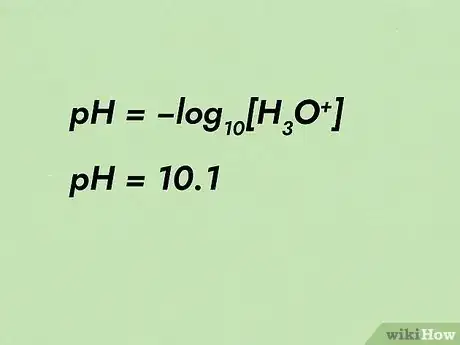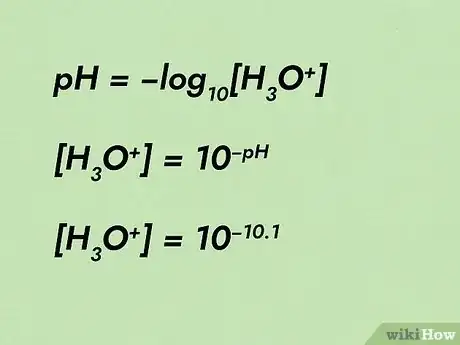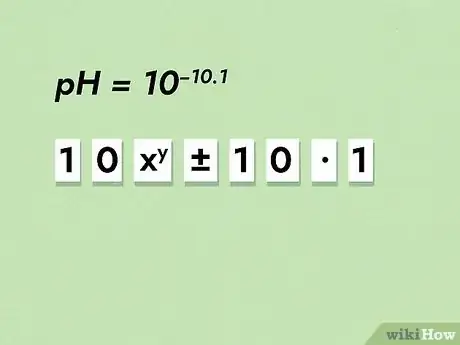This article was co-authored by Chris Hasegawa, PhD. Dr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
There are 10 references cited in this article, which can be found at the bottom of the page.
This article has been viewed 342,044 times.
You can calculate the pH of a chemical solution, or how acidic or basic it is, using the pH formula: pH = -log10[H3O+]. Anything less than 7 is acidic, and anything greater than 7 is basic. Check out the steps below to learn how to find the pH of any chemical solution using the pH formula.
Steps
Understanding pH
-
1Know what pH actually is. The pH is a measure of the concentration of hydrogen ions in a solution.[1] A solution with a high concentration of hydrogen ions is acidic. A solution with a low amount of hydrogen ions is basic, or also known as alkaline. Hydrogen ions, also known as hydronium, are written shorthand as H+ or H3O+.[2]
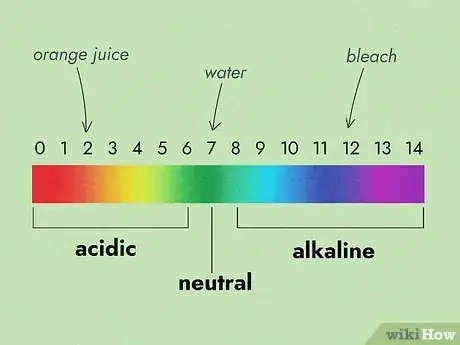
- Know the pH scale. The pH scale is usually presented from 0 to 14. The lower the number, the more acidic the solution. The higher the number, the more basic the solution. For example, orange juice would have a pH of 2 because it is quite acidic. In contrast, bleach has a pH of 12 as it is quite basic. Numbers in the middle of the scale are neutral, such as water, with a pH of 7.
- One level of pH is a 10x difference. For example, when comparing pH 7 to pH 6, pH 6 is ten times more acidic than pH 7. Furthermore, pH 6 would be 100 times more acidic than pH 8.
-
2Define pH in an equation. The pH scale is calculated by a negative logarithm. A negative logarithm of base b is simply how many times a number must be divided by b to reach 1.[3] The pH equation can be seen as follows: pH = -log10[H3O+].[4]
- The equation can sometimes be seen as pH = -log10[H+]. Know that whether the equation has H3O+ or H+, they are the same.
- It is not vital to have a firm understanding of what a negative log is to calculate pH. Most calculators used at high school and post secondary level will have a log button.
Advertisement -
3Understand concentration. Concentration is the number of particles of a compound in a solution relative to the volume of the same solution.[5] For pH, you have to use molar concentration for the formula to work out. Molar concentration, which is also called molarity, denotes the number of moles of dissolved compound per liter of solution. Its units are moles per liter (mol/L), also called molar (M). If you’re using a solution in a lab, the concentration will be written on the bottle. When working on your chemistry homework, the concentration will usually be given to you.[6]
Using the Concentration to Calculate pH
-
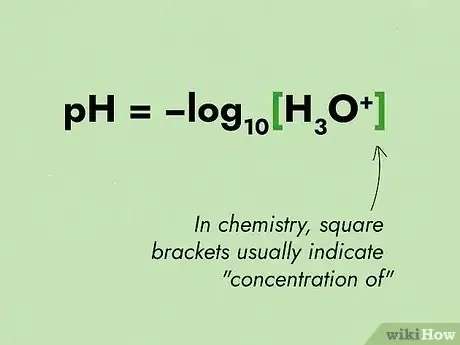
1Remember the pH equation. The pH equation is as follows: pH = -log10[H3O+].[7] Ensure you know what all terms in the equation represent. Look at which term is used for concentration.
- In chemistry, square brackets usually indicate "concentration of". So the equation of pH would be read as "pH equals the negative logarithm of the concentration of hydronium ions".
-
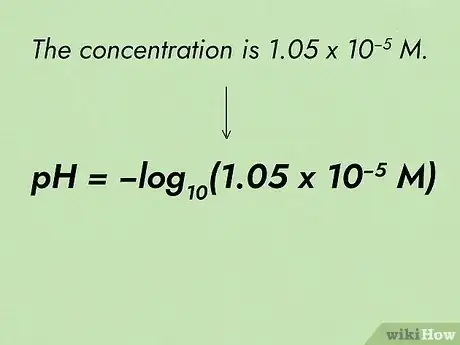
2Identify the actual concentration. Read over your chemistry question. Identify the concentration of the acid or base. Write down the entire equation on paper with the known values represented in the equation.[8] Always include units to avoid confusion.
- For example, if the concentration is 1.05 x 10-5 M, write the pH equation as: pH = -log10(1.05 x 10-5 M)
-
3Solve the equation. When solving the pH equation, you must use a scientific calculator. First, hit the “negative” button. It is usually written as “+/-”. Now key in the “log” button. Your screen should display “-log”.[9] Now hit an open bracket and enter in your concentration. Don’t forget to add exponents when necessary.[10] Follow with a closed bracket.[11] At this point, you should see “-log(1.05x10^-5). Hit solve. Your pH should be approximately 4.98.
Using pH to Calculate a Concentration
-
1Identify the known unknowns. First write out the pH equation. Next, identify the values you have by writing them directly below your equation. For example, if you know the pH is a value of 10.1, write it on the paper below the pH equation.
-
2Rearrange the equation. Rearranging the equation will require a strong understanding of algebra. To calculate concentration from pH, you must understand that the inverse of log10 is "10 to the power of ..." Start by shifting the minus sign over from the log side to the pH side. Then raise 10 to the power of (each side). "10 to the power of" and log10 are inverses of each other and cancels out.[12]
- For example, pH = -log10[H3O+] will mold into [H3O+] = 10-pH. pH can then be filled in as 10.1
-
3Solve the equation. When working with inverse log, the calculator process is unique. Remember that log is a type of multiplication by 10. To enter your equation, key in 10. Next, hit the “EXP” exponent button. Key in the negative sign followed by the value. Hit solve.
- For example, take a pH value of 10.1. Key in “10” followed by “EXP.” Now key in “-/+” to have our value be negative. Finally, key in the pH of “10.1”. Hit solve. You should get about 7.943ᴇ-11, or 7.943*10-11. This means our concentration is 7.943*10-11 M.
-
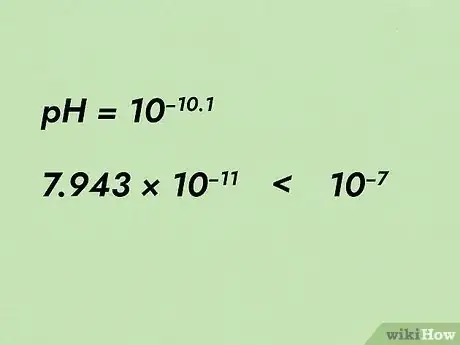
4Think about your answer. A neutral pH of 7 equates to a hydronium ion concentration of 10-7 M. A solution with a pH of 10.1 is basic, so it will have less hydronium ions than that. If we look at our answer, 7.943*10-11, we do indeed see that this number is way smaller than 10-7, so our answer does make sense.[13]
Expert Q&A
-
QuestionWhat are some natural indicators?
 Chris Hasegawa, PhDDr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
Chris Hasegawa, PhDDr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
Retired Science Professor & Dean Cabbage juice, celeries, and different kinds of flowers are all excellent, natural base indicators.
Cabbage juice, celeries, and different kinds of flowers are all excellent, natural base indicators. -
QuestionWhat is the purpose of a titration lab?
 Chris Hasegawa, PhDDr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
Chris Hasegawa, PhDDr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
Retired Science Professor & Dean Titration labs let you titrate an unknown acid solution with a basic solution that you already know the pH and molarity of.
Titration labs let you titrate an unknown acid solution with a basic solution that you already know the pH and molarity of. -
QuestionWhat is the most accurate method of reading pH?
 Chris Hasegawa, PhDDr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
Chris Hasegawa, PhDDr. Chris Hasegawa was a Science Professor and the Dean at California State University Monterey Bay. Dr. Hasegawa specializes in teaching complex scientific concepts to students. He holds a BS in Biochemistry, a Master’s in Education, and his teaching credential from The University of California, Davis. He earned his PhD in Curriculum and Instruction from The University of Oregon. Before becoming a professor, Dr. Hasegawa conducted biochemical research in Neuropharmacology at the National Institute of Health. He also taught physical and life sciences and served as a teacher and administrator at public schools in California, Oregon, and Arizona.
Retired Science Professor & Dean A pH meter is the most accurate options, but most people don't have those lying around at home. pH papers and other indicators can work, too.
A pH meter is the most accurate options, but most people don't have those lying around at home. pH papers and other indicators can work, too.
References
- ↑ Chris Hasegawa, PhD. Retired Science Professor & Dean. Expert Interview. 29 July 2021.
- ↑ https://www.clemson.edu/extension/food/canning/canning-tips/44what-is-ph.html
- ↑ https://www.mathsisfun.com/algebra/logarithms.html
- ↑ https://www.chem.purdue.edu/gchelp/howtosolveit/Equilibrium/Calculating_pHandpOH.htm
- ↑ http://www.gcsescience.com/m28.htm
- ↑ https://www.omnicalculator.com/chemistry/molarity
- ↑ http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch17/ph.php
- ↑ http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch17/ph.php
- ↑ http://www.chemteam.info/AcidBase/pH.html
About This Article
To calculate pH, remember that the pH scale goes from 0 to 14 with numbers below 7 being acidic and numbers above 7 being basic. If you are doing chemistry in a lab, you will need to determine the concentration by finding the moles per unit of volume (m/v or M). If you are doing a chemistry problem, look at the equation to identify the concentration. Then, use the formula pH = -log10[H3O+], where H equals Hydrogen ions, to find the pH. To learn how to use pH to calculate a concentration, keep reading!