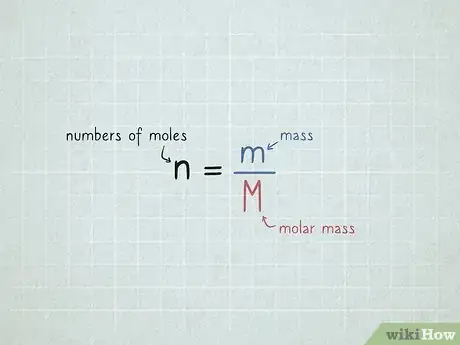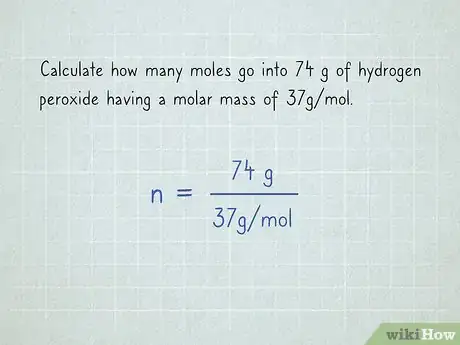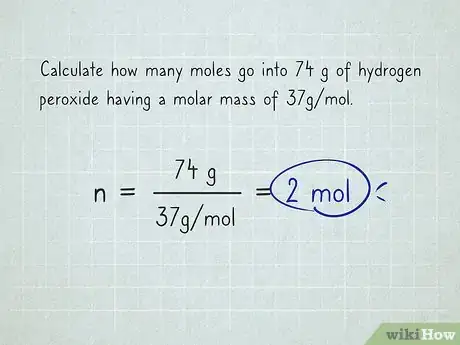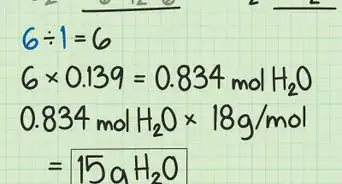X
wikiHow is a “wiki,” similar to Wikipedia, which means that many of our articles are co-written by multiple authors. To create this article, 11 people, some anonymous, worked to edit and improve it over time.
This article has been viewed 27,643 times.
Learn more...
Being able to calculate how many moles are in a certain amount of matter is an important skill in both inorganic and organics chemistry. In short, plug in any given values into the equation and solve for .
Steps
-
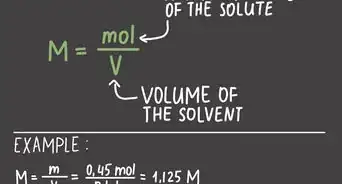
1Set up your equation. The equation you'll need to use is , or the numbers of moles is equal to the mass divided by the molar mass .[1]
-
2Substitute any given values. For example, if you have to calculate how many moles go into 74 g of hydrogen peroxide, you'll get , since you have 74 g of hydrogen peroxide, and the molar mass is 37 .[2]Advertisement
-
3Solve the equation and cancel out the units. The unit grams is both in the numerator and denominator so that cancels out. Moles is in the denominator of the denominator, which leaves you with moles in the numerator. The final answer is .[3]
Advertisement
Community Q&A
-
QuestionHow do I convert 8 grams of Hydrogen into moles?
 Community AnswerNumber of moles = mass / molecular mass = 8 / 1 = 8, knowing that the molecular mass of hydrogen is one.
Community AnswerNumber of moles = mass / molecular mass = 8 / 1 = 8, knowing that the molecular mass of hydrogen is one.
Advertisement
References
- ↑ https://www.omnicalculator.com/chemistry/mole
- ↑ https://www.omnicalculator.com/chemistry/mole
- ↑ https://chem.libretexts.org/Courses/Sacramento_City_College/SCC%3A_CHEM_330_-_Adventures_in_Chemistry_(Alviar-Agnew)/05%3A_Chemical_Accounting/5.04%3A_Molar_Mass-_Mole-to-Mass_and_Mass-to-Mole_Conversions
About This Article
Advertisement