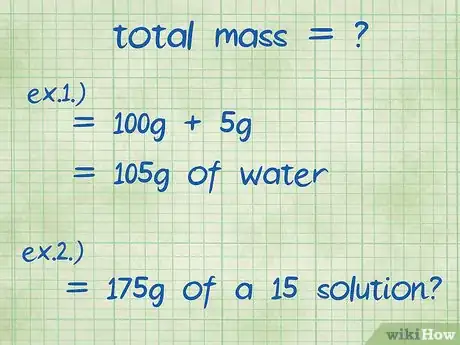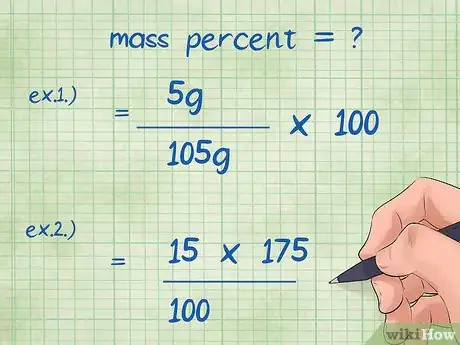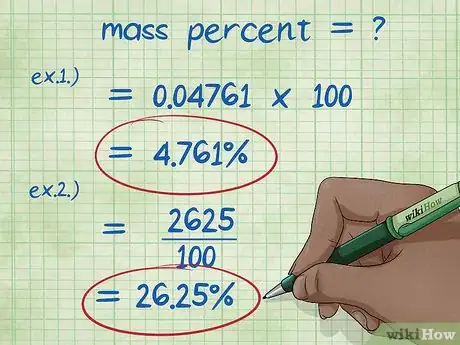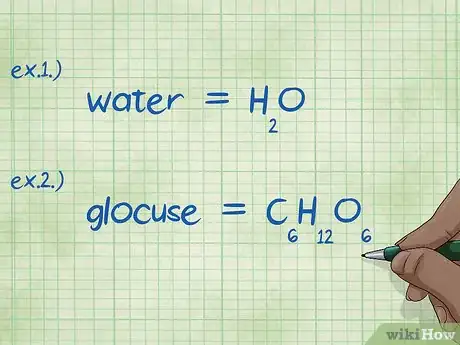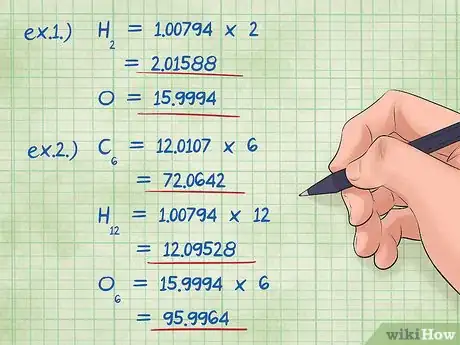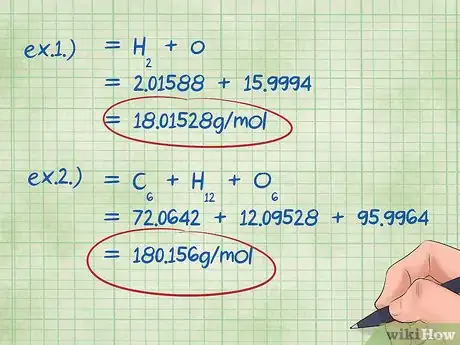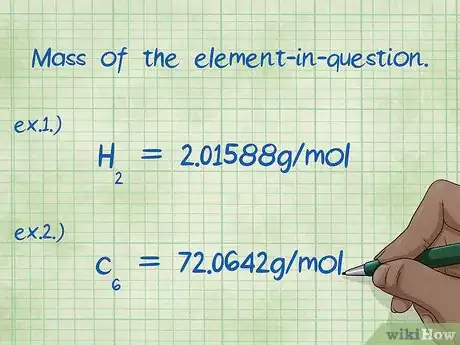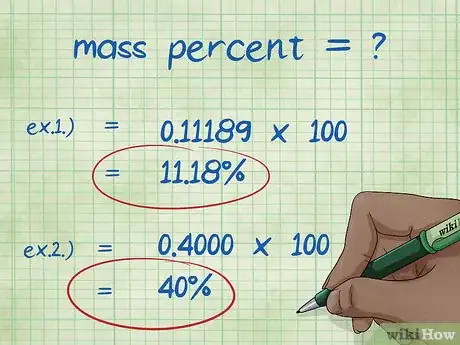This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
There are 7 references cited in this article, which can be found at the bottom of the page.
wikiHow marks an article as reader-approved once it receives enough positive feedback. In this case, several readers have written to tell us that this article was helpful to them, earning it our reader-approved status.
This article has been viewed 618,243 times.
Mass percent tells you the percentage of each element that makes up a chemical compound. Finding the mass percent requires the molar mass of the elements in the compound in grams/mole or the number of grams used to make a solution.[1] It is simply calculated using a basic formula dividing the mass of the element (or solute) by the mass of the compound (or solution).
Steps
Solving for Mass Percent When Given Masses
-
1Define the equation for mass percent of a compound. The basic formula for mass percent of a compound is: mass percent = (mass of chemical/total mass of compound) x 100. You must multiply by 100 at the end to express the value as a percentage.[2]
- Write the equation at the beginning of every problem: mass percent = (mass of chemical/total mass of compound) x 100.
- Both of the values should be in grams so that they cancel each other out once you solve the equation.
- The mass of the chemical you’re interested in is the mass given in the problem. If the mass isn’t given, refer to the following section about solving for mass percent when the mass is not given.
- The total mass of the compound is calculated by summing the masses of all of the chemicals used to make the compound or solution.
-
2Calculate the total mass of the compound. When you know the masses of all the elements or compounds being added together, you simply need to add them together to calculate the total mass of the final compound or solution. This will be the denominator in the mass percent calculation.[3]
- Example 1: What is the percent mass of 5g of sodium hydroxide dissolved in 100g of water?
- The total mass of the compound is the amount of sodium hydroxide plus the amount of water: 100g + 5g for a total mass of 105g.
- Example 2: What masses of sodium chloride and water are needed to make 175 g of a 15% solution?
- In this example, you are given the total mass and the percentage you want, but are asked to find the amount of solute to add to the solution. The total mass is 175 g.
Advertisement - Example 1: What is the percent mass of 5g of sodium hydroxide dissolved in 100g of water?
-
3Identify the mass of the chemical-in-question. When asked to find the "mass percent", you are being asked to find the mass of a particular chemical (the chemical-in-question) as a percentage of the total mass of all elements. Write down the mass of chemical-in-question. This mass will be the numerator in the mass percent calculation.
- Example 1: The mass of the chemical-in-question is 5g of sodium hydroxide.
- Example 2: For this example, the mass of the chemical-in-question is the unknown you are trying to calculate.
-
4Plug the variables into the mass percent equation. Once you have determined the values for each variable, plug them into the equation.[4]
- Example 1: mass percent = (mass of chemical/total mass of compound) x 100 = (5 g/105 g) x 100.
- Example 2: We want to rearrange the mass percent equation to solve for the unknown mass of the chemical: mass of the chemical = (mass percent*total mass of the compound)/100 = (15*175)/100.
-
5Calculate the mass percent. Now that the equation is filled in, simply solve to calculate the mass percent. Divide the mass of the chemical by the total mass of the compound and multiply by 100. This will give you the mass percent of the chemical.[5]
- Example 1: (5/105) x 100 = 0.04761 x 100 = 4.761%. Thus, the mass percent of 5g of sodium hydroxide dissolved in 100g of water is 4.761%.
- Example 2: The rearranged equation to solve for mass of the chemical is (mass percent*total mass of the compound)/100: (15*175)/100 = (2625)/100 = 26.25 grams sodium chloride.
- The amount of water to be added is simply the total mass minus the mass of the chemical: 175 – 26.25 = 148.75 grams water.
Solving for Percent Mass When Not Given Masses
-
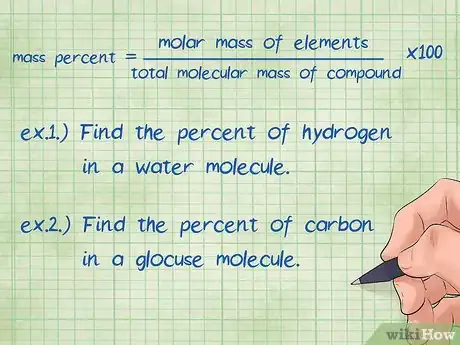
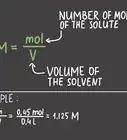
1Define the equation for mass percent of a compound. The basic formula for mass percent of a compound is: mass percent = (molar mass of element/total molecular mass of compound) x 100. The molar mass of a compound is the amount of mass for one mole of an element while the molecular mass is the amount of mass for one mole of the entire compound.[6] You must multiply by 100 at the end to express the value as a percentage.[7]
- Write out the equation at the beginning of every problem: mass percent = (molar mass of element/total molecular mass of compound) x 100.
- Both values have units of grams per mole (g/mol). This means the units will cancel each other out when you solve the equation.
- When you aren’t given masses, you can find the mass percent of an element within a compound using molar mass.
- Example 1: Find the mass percent of Hydrogen in a water molecule.
- Example 2: Find the mass percent of carbon in a glucose molecule.
-
2Write out the chemical formula. If you are not given the chemical formulas for each compound, you will need to write them out. If you are given the chemical formulas you may skip this step, and proceed to the "Find the mass of each element" step.
- Example 1: Write out the chemical formula for water, H2O.
- Example 2: Write out the chemical formula for glucose, C6H12O6.
-
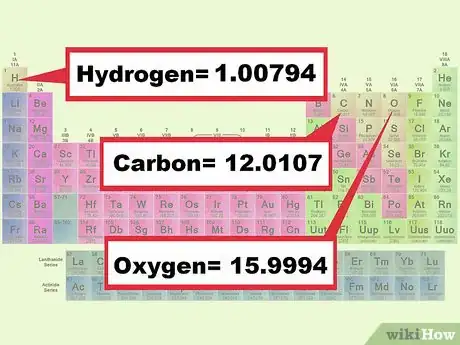
3Find the mass of each element in the compound. Look up the molecular weight of each element in your chemical formulas on the periodic table. The mass of an element can usually be found underneath the chemical symbol. Write down the masses of every element in the compound.[8]
- Example 1: Look up the molecular weight of Oxygen, 15.9994; and the molecular weight of Hydrogen, 1.0079.[9]
- Example 2: Look up the molecular weight of Carbon, 12.0107; Oxygen, 15.9994; and Hydrogen, 1.0079.
-
4Multiply the masses by the mole ratio. Identify how many moles (mole ratio) of each element are in your chemical compounds. The mole ratio is given by the subscript number in the compound. Multiply the molecular mass of each element by this mole ratio.
- Example 1: Hydrogen has a subscript of two while oxygen has a subscript of 1. Therefore, multiply the molecular mass of Hydrogen by 2, 1.00794 X 2 = 2.01588; and leave the molecular mass of Oxygen as is, 15.9994 (multiplied by one).
- Example 2: Carbon has a subscript of 6, hydrogen, 12, and oxygen, 6. Multiplying each element by its subscript gives you:
- Carbon (12.0107*6) = 72.0642
- Hydrogen (1.00794*12) = 12.09528
- Oxygen (15.9994*6) = 95.9964
-
5Calculate the total mass of the compound. Add up the total mass of all the elements in your compounds. Using the masses calculated using the mole ratio, you can calculate the total mass of the compound. This number will be the denominator of the mass percent equation.[10]
- Example 1: Add 2.01588 g/mol (the mass of two moles of Hydrogen atoms) with 15.9994 g/mol (the mass of a single mole of Oxygen atoms) and get 18.01528 g/mol.
- Example 2: Add all of the calculated molar masses together: Carbon + Hydrogen + Oxygen = 72.0642 + 12.09528 + 95.9964 = 180.156 g/mol.
-
6Identify the mass of the element-in-question. When asked to find the "mass percent", you are being asked to find the mass of a particular element in a compound, as a percentage of the total mass of all elements. Identify the mass of the element-in-question and write it down. The mass is the mass calculated using the mole ratio. This number is the numerator in the mass percent equation.[11]
- Example 1: The mass of hydrogen in the compound is 2.01588 g/mol (the mass of two moles of hydrogen atoms).
- Example 2: The mass of carbon in the compound is 72.0642 g/mol (the mass of six moles of carbon atoms).
-
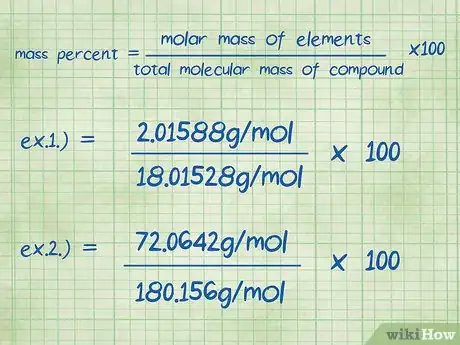
7Plug the variables into the mass percent equation. Once you have determined the values for each variable, plug them into the equation defined in the first step: mass percent = (molar mass of the element/total molecular mass of compound) x 100.[12]
- Example 1: mass percent = (molar mass of the element/total molecular mass of compound) x 100 = (2.01588/18.01528) x 100.
- Example 2: mass percent = (molar mass of the element/total molecular mass of compound) x 100 = (72.0642/180.156) x 100.
-
8Calculate the mass percent. Now that the equation is filled in, simply solve to calculate the mass percent. Divide the mass of the element by the total mass of the compound and multiply by 100. This will give you the mass percent of the element.[13]
- Example 1: mass percent = (2.01588/18.01528) x 100 = 0.11189 x 100 = 11.18%. Thus, the mass percent of Hydrogen atoms in a water molecule is 11.18%.
- Example 2: mass percent = (molar mass of the element/total molecular mass of compound) x 100 = (72.0642/180.156) x 100 = 0.4000 x 100 = 40.00%. Thus, the mass percent of carbon atoms in a molecule of glucose is 40.00%.
Expert Q&A
Did you know you can get expert answers for this article?
Unlock expert answers by supporting wikiHow
-
QuestionIf a solution is prepared by mixing 50 ml of vinegar into 300 ml of solution, what is the percentage by volume?
 Bess Ruff, MABess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
Bess Ruff, MABess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
Environmental Scientist You can use the same formula to calculate percentage by volume as you use to calculate percentage by mass. First, calculate the total volume of the solution. Then, divide the volume of the vinegar by the total volume of the solution. Finally, multiply by 100 to get the percentage of vinegar in the total solution. 50 ml + 300 ml = 350 ml for the total volume of the solution. Vinegar percentage = 50/350 = 14 x 100 = 14% vinegar.
You can use the same formula to calculate percentage by volume as you use to calculate percentage by mass. First, calculate the total volume of the solution. Then, divide the volume of the vinegar by the total volume of the solution. Finally, multiply by 100 to get the percentage of vinegar in the total solution. 50 ml + 300 ml = 350 ml for the total volume of the solution. Vinegar percentage = 50/350 = 14 x 100 = 14% vinegar. -
QuestionHow do I determine the mass percentage of salt (NaCl) in a water-salt solution?
 Community AnswerIn order to calculate the mass percentage, you must know how much salt was added to a certain amount of water. For instance, if you added 50 grams of NaCl to 1000 grams of water, that mass percent would be 50/1050 x 100 = 4.76%.
Community AnswerIn order to calculate the mass percentage, you must know how much salt was added to a certain amount of water. For instance, if you added 50 grams of NaCl to 1000 grams of water, that mass percent would be 50/1050 x 100 = 4.76%. -
QuestionHow would I calculate the mass percent of a MgBr2 solution that contains 5.80g of MgBr2 in 45.0g of solution?
 Community AnswerSimilar to the example given above, simply plug the numbers into the mass percent equation. Calculate total mass of the compound: 5.8 + 45 = 50.8. Divide mass of the chemical by mass of the compound and multiply by 100: 5.8/50.8 x 100 = 11.42%.
Community AnswerSimilar to the example given above, simply plug the numbers into the mass percent equation. Calculate total mass of the compound: 5.8 + 45 = 50.8. Divide mass of the chemical by mass of the compound and multiply by 100: 5.8/50.8 x 100 = 11.42%.
References
- ↑ https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Quantifying_Nature/Density_and_Percent_Compositions
- ↑ https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Map%3A_Introductory_Chemistry_(Tro)/06%3A_Chemical_Composition/6.06%3A_Mass_Percent_Composition_of_Compounds
- ↑ https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Map%3A_Introductory_Chemistry_(Tro)/06%3A_Chemical_Composition/6.06%3A_Mass_Percent_Composition_of_Compounds
- ↑ https://www.omnicalculator.com/chemistry/mass-percent
- ↑ https://www.omnicalculator.com/chemistry/mass-percent
- ↑ http://www.webqc.org/mmcalc.php
- ↑ https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Quantifying_Nature/Density_and_Percent_Compositions
- ↑ https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Map%3A_Introductory_Chemistry_(Tro)/06%3A_Chemical_Composition/6.07%3A_Mass_Percent_Composition_from_a_Chemical_Formula
- ↑ http://sciencenotes.org/wp-content/uploads/2015/01/Periodic-Table-Color.png
- ↑ https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Map%3A_Introductory_Chemistry_(Tro)/06%3A_Chemical_Composition/6.07%3A_Mass_Percent_Composition_from_a_Chemical_Formula
- ↑ https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Map%3A_Introductory_Chemistry_(Tro)/06%3A_Chemical_Composition/6.07%3A_Mass_Percent_Composition_from_a_Chemical_Formula
- ↑ https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Map%3A_Introductory_Chemistry_(Tro)/13%3A_Solutions/13.05%3A_Specifying_Solution_Concentration-_Mass_Percent
- ↑ https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Map%3A_Introductory_Chemistry_(Tro)/06%3A_Chemical_Composition/6.07%3A_Mass_Percent_Composition_from_a_Chemical_Formula
About This Article
To calculate mass percent, start by identifying the mass of the chemical-in-question. Next, figure out the total mass of the compound by adding together the masses of all of the chemicals used to make that compound. Then, divide the mass of the chemical by the total mass of compound. Multiply the answer you get by 100 to calculate the percentage! To learn how to calculate mass percent when you don't know any of the masses involved, read on!