This article was co-authored by Meredith Juncker, PhD. Meredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
There are 9 references cited in this article, which can be found at the bottom of the page.
This article has been viewed 34,628 times.
Maybe you want to denature a protein for a science project, or maybe you've read about denatured food and want to know how that works. Denaturation is the process by which a protein loses its shape and structure due to actions from external forces, including heat, radiation, acids, and solvents. Organic solvents usually denature proteins.[1] The exact way you denature a protein depends on the particular one you want to work with, since they all require different denaturing agents at different levels. Most any protein can be denatured, though, if you know what to change in the protein's environment.
Steps
Denaturing a Protein
-
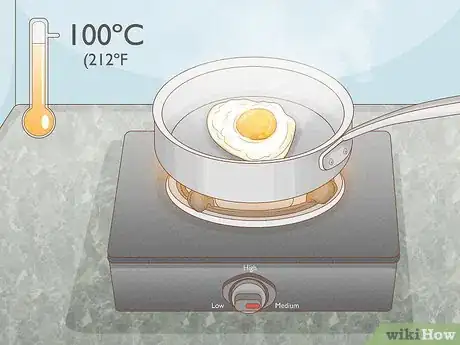
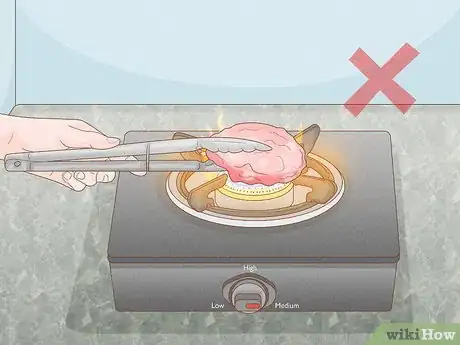
1Use heat. Heat is one of the easiest ways and most common ways to denature a protein. When the protein in question is present in food, simply cooking the food will denature the proteins. Many proteins can be denatured by exposing them to a temperature of or above 100° C (212° F).[2] This allows the proteins to coagulate and reduce their solubility.
- The length of exposure depends on what you are exposing to heat. An egg, for example, may cook in a pan on medium heat in five minutes, while a roast may take hours to cook in an oven.
-
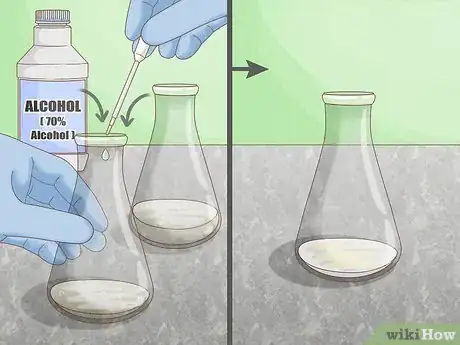
2Apply alcohol. Alcohol disrupts the hydrogen bonds that occur between amide groups of a peptide bond. When a protein is exposed to an alcohol solution, the alcohol molecules form new bonds with the protein chain. Use a solution of 70% alcohol to break through the cell wall of bacteria and denature the protein.[3]
- Concentrated alcohols can be dangerous, as they are both flammable and toxic. Always wear full safety equipment including gloves and eye protection, and handle in a secure, temperature-controlled environment.
Advertisement -
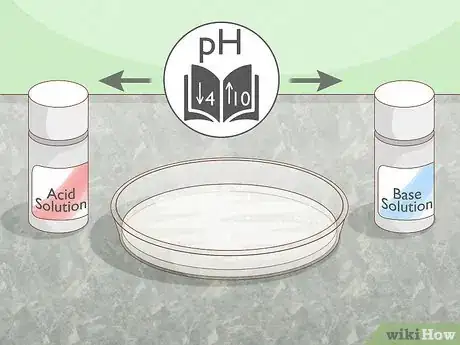
3Change the pH. The internal structure of a protein can be broken down when its environment is very acidic or very alkaline, as they disrupt the ionic bonds that hold the protein’s salt bridges together. Add an acid or base solution to the protein. The surrounding environment should be at a pH of above 10 or below 4 to encourage denaturation.[4] If you're inducing the change with acid, the pH should be between 2 and 5.[5]
- Changing the pH can ionize an amino acid, depending on the pKa of each functional group in that amino acid. You can also ionize the amino group or carboxyl group present in the amino acid.
-
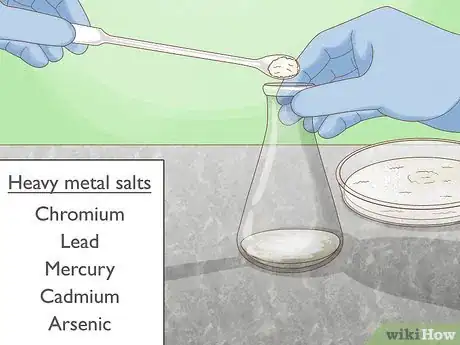
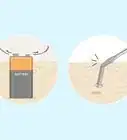
4Try heavy metal salts. Heavy metals can disrupt bonds in the protein, causing it to lose its structure. Salts of heavy metals such as mercury and lead may be used to denature different proteins. Such salts are available from most chemical suppliers, and should always be used with caution and proper safety equipment including gloves and eye protection.[6]
- Heavy metals can interact with a protein's functional side chain groups to form complexes. Heavy metals also oxidize the protein's amino acid side chains.[7]
Renaturing Proteins
-
1Determine if a protein can refold. Some forms of denaturation are permanent, while others can be undone. Cooking an egg or meat, for example, cannot be undone, but a protein that has been exposed to a high pH may regain shape when put in a more neutral environment.[8]
- Whether or not the protein can refold will depend on its DNA. The DNA will hold the information needed for the protein to return to its native state.[9]
-
2Remove the denaturing factor. Return the environment around the protein to stasis and remove the denaturing element. Remove the acid or base, for example, or bring the protein back to a more reasonable temperature.[10]
-
3Use a renaturation kit. Many laboratory supplies companies sell renaturation kits that allow you to screen for the best parameters to encourage renaturation. Such kits can be especially helpful if you are looking at proteins in a laboratory or experimental setting.
References
- ↑ https://www.education.com/science-fair/article/denaturing-proteins/
- ↑ http://bitesizebio.com/20794/the-nature-of-denaturing-protein-gels-that-is/
- ↑ http://chemistry.elmhurst.edu/vchembook/568denaturation.html
- ↑ http://www.chemistryexplained.com/Co-Di/Denaturation.html
- ↑ https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470015902.a0003004.pub2
- ↑ https://www.ncbi.nlm.nih.gov/pubmed/18501191
- ↑ http://www.mdpi.com/2218-273X/4/1/252
- ↑ http://www.biology-pages.info/D/DenaturingProtein.html
- ↑ https://www.science.org/doi/10.1126/science.181.4096.223