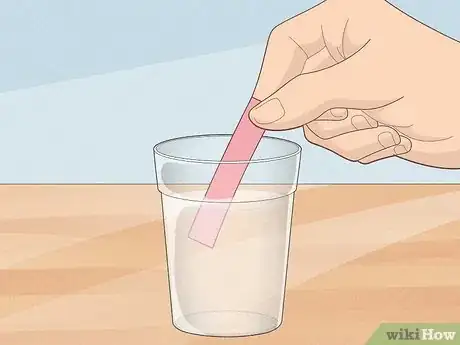This article was co-authored by Meredith Juncker, PhD. Meredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
There are 9 references cited in this article, which can be found at the bottom of the page.
This article has been viewed 549,552 times.
Testing the pH of water tells you how acidic or basic the water is at the moment of testing. Pure, unpolluted water normally has a pH level of 7, which is neutral (neither acidic nor basic). The pH level of water can provide information on potential contamination and can be an important precaution for protecting the health of people, animals, and vegetation.
Steps
Using a pH Meter
-
1Calibrate the probe and meter following the manufacturer specifications. You may need to calibrate the meter by testing it in a substance with a known pH rating. You can then adjust the meter accordingly. If you will be testing water away from a lab, you may want to perform this calibration several hours before you take the meter to the field.[1]
- Rinse the probe with double deionized water before using it. Dry it off with a clean tissue.
-
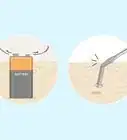
2Collect a sample of the water in a clean container. The water sample must be deep enough to cover the tip of the electrode. Let the sample sit for a moment so the temperature can stabilize, then measure the temperature of the sample using a thermometer.Advertisement
-
3Adjust the meter to match the sample temperature. The probe's sensitivity is affected by the temperature of the water, and so the reading of the meter cannot be accurate if you do not input the temperature data. The pH of the water will also be affected by the water’s temperature—pure water has a lower pH at higher temperatures and a higher pH at lower temperatures.[2]
-
4Put the probe into the sample. Wait for the meter to come to equilibrium. The meter has reached equilibrium when the measurement becomes steady.[3]
-
5Read the pH measurement of the sample. Your pH meter should provide a reading on the scale of 0-14. If the water is pure, it should read close to 7. Record your findings.[4]
- A pH reading lower than 7 indicates that the water is acidic, while a reading higher than 7 indicates that the water is basic.
Using Litmus Papers
-
1Get to know the difference between pH paper and litmus paper. To obtain an accurate reading of a solution, you can use pH paper. This is not to be confused with the common litmus paper. Both can be used to test for acids and bases, but they differ in important ways. pH paper will tell you the actual pH value of the water, but litmus strips typically only indicate whether the water is basic or acidic.[5]
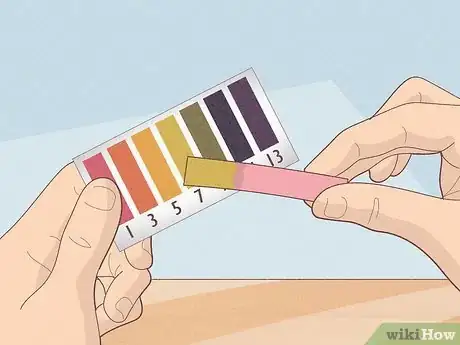
- pH strips contain a series of indicator bars that will all change color after exposure to a solution. The strength of the acids and bases on each bar differs. After they change, the color pattern of the bars can be matched to the examples that come with the kit.
- Litmus papers are strips of paper that contain an acid or a base (alkaline). The most common of these are red (which contains an acid that reacts with bases) and blue (which contains a base that reacts with acids). The red strips turn blue if the substance is alkaline, and the blue strips turn red if they contact an acid. Litmus papers can be used to provide a quick and easy test, but the cheapest of them do not always provide accurate readings on the strength of the solution.
-
2Collect a sample of the water in a clean container. The water sample must be deep enough to cover the test strip.
-
3Dip a test strip into your sample. Just a few seconds of exposure will suffice. The different indicator bars on the paper will begin changing color within a few moments.[6]
-
4Compare the test strip with the color chart that came with the paper. The color(s) on the chart should match the color(s) of your test strip. The chart should correlate color patterns to pH levels.
Understanding pH
-
1Learn about how acids and bases are defined. Acidity and alkalinity (the term used to describe bases) are both defined by the hydrogen ions they lose or accept. An acid is a substance that loses (or, some say, donates) hydrogen ions. A base is a substance that accepts additional hydrogen ions.[7]
-
2Familiarize yourself with the pH scale. The pH number is used to rate the level of acidity or alkalinity of water-soluble substances. Water normally contains an equal amount of hydroxide ions (OH-) and hydronium ions (H30+). When an acidic or alkaline substance is added to water, they change the proportion of hydroxide and hydronium ions.[8]
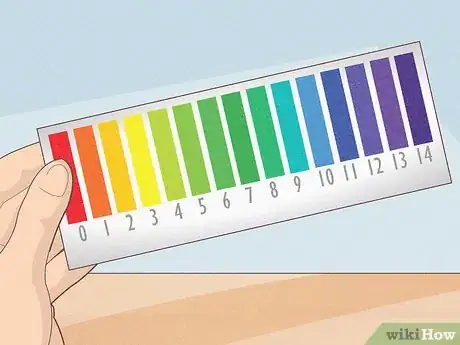
- pH is typically measured on a scale of 0 to 14 (though substances can actually be far beyond that range). Neutral substances rate close to 7, acidic ones below 7, and alkaline substances are above 7.
- The pH scale is a logarithmic scale, meaning that differences of a single integer actually represent a tenfold difference in acidity or alkalinity. For instance, a substance that has a pH of 2 is actually 10 times more acidic than one with a pH of 3 and 100 times more acidic than a substance with a pH of 4. The scale functions similarly for alkaline substances, with 1 integer representing a tenfold difference in alkalinity.
-
3Get to know why we test the pH of water. Pure water should have a pH of 7, though common tap water has a pH between 6 and 5.5. Highly acidic water (water with a low pH) is more likely to dissolve toxic chemicals. These can contaminate the water and make it unsafe for human consumption.[9]
- It is often considered best to test pH on site. If you collect a water sample for study in a lab, carbon dioxide in the air can dissolve into the water. The dissolved carbon dioxide reacts with the ions in the water to increase acidity in basic or neutral solutions. To prevent carbon dioxide contamination, you must test water within 2 hours of collection.[10]
Expert Q&A
Did you know you can get premium answers for this article?
Unlock premium answers by supporting wikiHow
-
QuestionHow effective is litmus paper?
 Meredith Juncker, PhDMeredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
Meredith Juncker, PhDMeredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
Scientific Researcher
-
QuestionWhich of the methods mentioned above is the best?
 Meredith Juncker, PhDMeredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
Meredith Juncker, PhDMeredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases.
Scientific Researcher
-
QuestionCan I make test strips for pH myself?
 wikiHow Staff EditorThis answer was written by one of our trained team of researchers who validated it for accuracy and comprehensiveness.
wikiHow Staff EditorThis answer was written by one of our trained team of researchers who validated it for accuracy and comprehensiveness.
Staff Answer wikiHow Staff EditorStaff AnswerSure, you can make your own test strips at home, and it’s not that hard to do. For help with making your own test strips, check out the wikiHow: How to Make Homemade pH Paper Test Strips.
wikiHow Staff EditorStaff AnswerSure, you can make your own test strips at home, and it’s not that hard to do. For help with making your own test strips, check out the wikiHow: How to Make Homemade pH Paper Test Strips.
References
- ↑ https://sciencing.com/calibrate-ph-meter-4796148.html
- ↑ https://sciencing.com/effects-temperature-ph-water-6837207.html
- ↑ https://personal.utdallas.edu/~brikowi/Teaching/Field_Methods/Using_pH_Meter.html
- ↑ https://personal.utdallas.edu/~brikowi/Teaching/Field_Methods/Using_pH_Meter.html
- ↑ https://sciencing.com/differences-between-litmus-paper-ph-strips-13673.html
- ↑ https://sciencing.com/use-litmus-papers-4745370.html
- ↑ https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Acids_and_Bases/Acid/Overview_of_Acids_and_Bases
- ↑ https://www.usgs.gov/media/images/ph-scale-0
- ↑ https://www.usgs.gov/special-topic/water-science-school/science/ph-and-water?qt-science_center_objects=0#qt-science_center_objects
About This Article
To test its pH, collect a sample of water in a small, clean container. Once you have your sample, prepare your probe by running it under clean water, or take your litmus paper out of its packaging. Then, place the test in the water. For litmus paper, a few seconds in the water will normally produce a clear reading. For a probe, wait until the reading on the screen is steady. Finally, compare the litmus paper to the chart on the packaging or read the measurement on the probe. For more tips on checking the pH of water, including how to understand your measurements, scroll down!