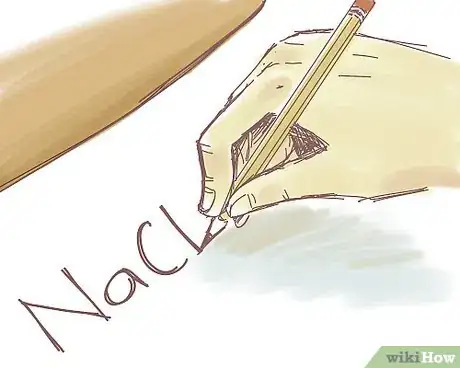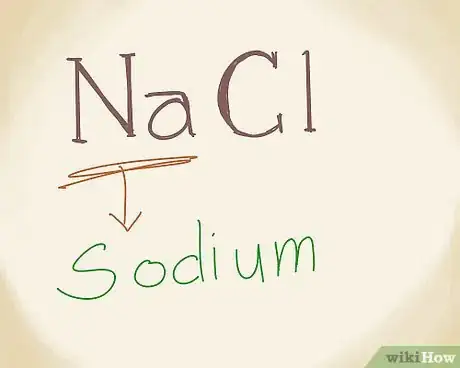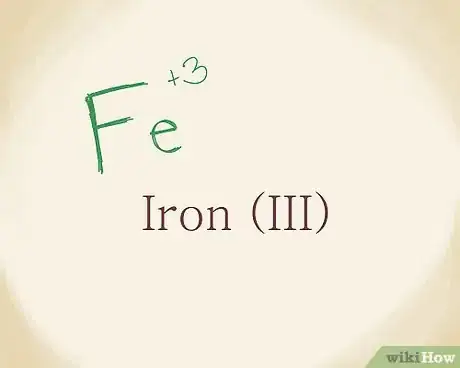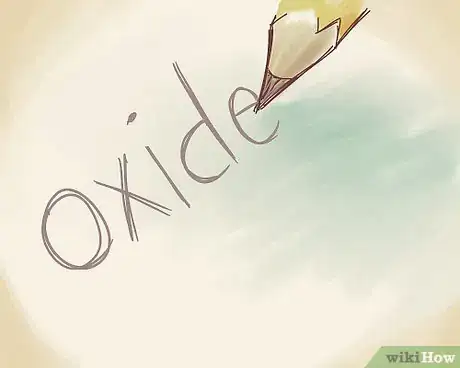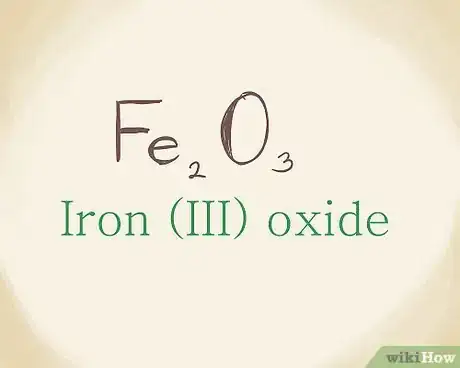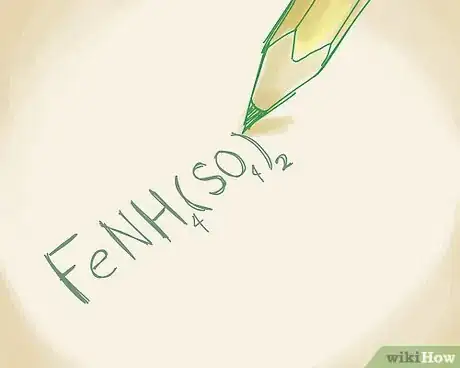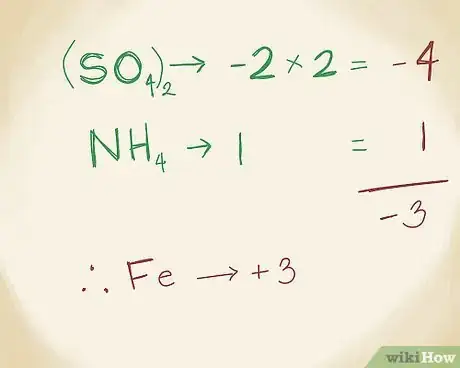This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
There are 8 references cited in this article, which can be found at the bottom of the page.
This article has been viewed 523,968 times.
Ionic compounds are a type of chemical compound made up of metal cations (positive ions) and non-metal anions (negative ions). To name an ionic compound, you simply need to find the names of the cation and anion present in the compound and make sure to revise the ends of metal names as needed. First, write out the name of the metal, followed by the name of the non-metal with its new ending. As an additional step, you'll need to calculate the charge of the metal ion if you're working with a transition metal.
Steps
Naming Basic Ionic Compounds
-
1Consult the periodic table of elements. When naming ionic compounds, all of the information you’ll need is on the periodic table. Ionic compounds are formed of a metal (cation) and a non-metal (anion). You can find metals on the left and central sections of the periodic table (e.g., Barium, Radium, and Lead), while you can find non-metals on the right side of the table.[1]
- Anions typically belong to groups 15, 16, or 17 on the periodic table.[2] Most versions of the periodic table will be color coded to indicate which elements are metal and which are non-metal.
- If you don’t have easy access to a copy of the table, access it online at: https://www.ptable.com/.
-
2Jot down the formula of the ionic compound. Let's say the ionic compound you're working with is NaCl. Use a pen or pencil to write this down on a sheet of paper. Or, if you're working in a classroom, write "NaCl" on the whiteboard.
- This is an example of a basic ionic compound. Basic compounds do not contain transition metals and contain only 2 ions.
Advertisement -
3Write the name of the metal. The first part of an ionic compound is called the "cation," which is a metal. This is the positively charged ion in the compound, and it is always written first in ionic compound formulas. Check the period table to find the name of “Na” if you need to. Na is sodium. So, write sodium.[3]
- Regardless of the kind of ionic compound you’re dealing with, the name of the metal is always written first.
-
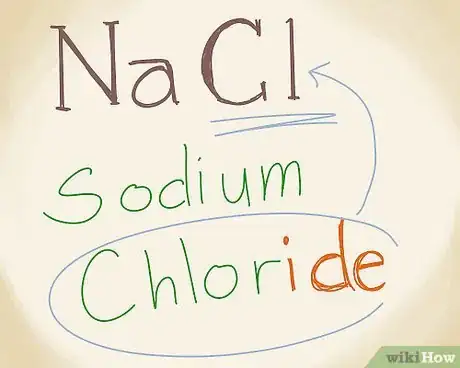
4Add the name of the non-metal with an –ide ending. The second component of an ionic compound is the non-metal anion. Write the non-metal's name with an "-ide" ending. Cl is chlorine. To add the "-ide" ending, just drop the 1 or 2 syllables ("-ine" in this case), and add "-ide" instead. Chlorine becomes chloride.[4]
- This naming principle applies to other anions as well. For example, in an ionic compound, “Phosphorus” becomes “Phosphide” and “Iodine” becomes “Iodide.”
-
5Combine the cation and anion names. Once you’ve found the names for the 2 components of the ionic compound, you’ve done nearly all of the work. Now you just need to put the parts together. NaCl can be written as sodium chloride.[5]
-
6Practice naming more simple ionic compounds. Once you've figured out how to name this ionic compound, try naming a few more simple ionic compounds. Memorizing a few common ionic compounds can help you have a better understanding of how to name ionic compounds. Remember that you don’t need to worry about the number of individual ions when naming the compounds. Here are a few more common ionic compounds:
- Li2S = Lithium sulfide
- Ag2S = Silver sulfide
- MgCl2 = Magnesium chloride
Naming Ionic Compounds with Transition Metals
-
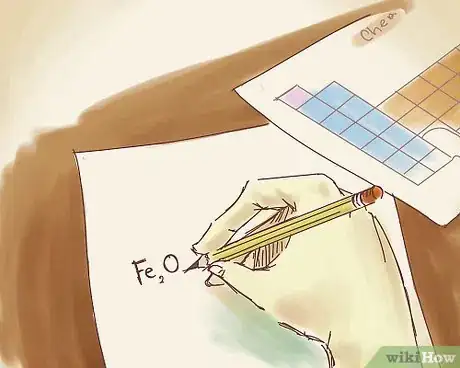
1Write down the formula of the ionic compound. For example, let's say you're working with this compound: Fe2O3. Transition metals can be found in the middle of the periodic tables and include metals like Platinum, Gold, and Zirconium. To account for this in the ionic compound’s name, you’ll need to insert a Roman numeral.[6]
- Transition metals take a little more work to name in ionic compounds, since their oxidation numbers (or their charges) are constantly changing.
-
2Find out the charge of the metal. If your metal comes from group 3 (or higher) in the periodic table, you’ll need to figure out its charge. The subscript numeral of the anion that the metal is paired with indicates the charge of a transition metal.[7] Metals will have a positive charge, so in this case, you’ll cross over the 3 from the O3 and write that Fe has a charge of +3.
- You can also do the reverse and write that O has a charge of -2.
- In many high-school- or college-level chemistry assignments, the metal’s charge will be provided for you.
-
3Name the metal and add a Roman numeral as needed. Consult the periodic table if you need to find out the chemical code for the metal you’re dealing with. Since Fe is iron and it has a charge of +3, you can write down Iron (III).[8]
- Remember to only use the Roman numeral when you're writing out the ionic compound’s name and not when writing the formula.
-
4Name the non-metal by revising the suffix. Refer to the periodic table if you forget the anion name. Since O is oxygen, you can drop the “–gen” ending and add the "-ide" ending. Call it "oxide."[9]
- Anions always take the –ide suffix. So, you’ll name anions the same regardless of what type of metal they’re paired with in an ionic compound.
-
5Combine the names to generate the ionic compound name. This part is no different from writing out the name of an ionic compound that doesn’t have a transition metal. Combine the metal and non-metal names (Roman numeral included) to name the ionic compound: Fe2O3 = Iron (III) oxide.[10]
-
6Use the older naming method instead of Roman numerals. Under the older naming method, you use "-ous" and "-ic" endings for transition metals instead of the Roman numerals. Look at the 2 ionic components of the compound. If the metal has a lower numerical charge than the non-metal, then you add the "-ous" ending. If the metal has a higher charge, then add an "-ic" ending.[11]
- Fe2+ has the lower state than oxygen (Fe3+ has the higher state), so “Fe” becomes ferrous. The name of Fe2+O can also be written as ferrous oxide.
- The terms “ferric” and “ferrous” are both use to refer to ions containing iron, since iron’s symbol is “Fe.”
-
7Don’t use Roman numerals when naming compounds with zinc or silver. The 2 transition metals that do have a definite charge are zinc (Zn) and silver (Ag). So, the charge of the metal in ionic compounds with zinc or silver doesn’t need to be borrowed from the subscript of the anion. Zinc always has a charge of +2 and silver always has a charge of +1.[12]
- This means that you don't have to use Roman numerals or the older naming method in describing those elements.
Naming Ionic Compounds with Polyatomic Ions
-
1Write the formula for the polyatomic ion. Polyatomic ionic compounds will have more than 2 ions in it. In most polyatomic compounds, 1 ion will be metal and the remainder will be non-metals. As always, refer to the periodic table to find the names of each ion. Let's say you're working with the following compound: FeNH4(SO4)2.[13]
-
2Find the charge of the metal. First, the SO4 ion has a charge of -2. You also know that there are two of these ions because of the “2” below the parenthesis. This ion is called “sulfate” because it’s a combination of oxygen and sulfur. So, 2 x -2 = -4. Then, the NH4, or the ammonium ion, has a charge of +1. You can figure out that it has this positive charge since ammonia itself is neutral, and ammonium contains 1 additional hydrogen molecule. (Ammonium is so-called because it combines 1 nitrogen molecule with 4 hydrogen molecules.) Add -4 and 1 and you get -3. This means that the iron ion, Fe, must have a charge of +3 to make up for it and to make the compound neutral.[14]
- Ionic compounds always have a neutral charge. You can use this information to calculate the metal’s charge.
- SO4 has a charge of -2 since it’s negative without the 2 hydrogen atoms that it had when it existed as sulfuric acid.[15]
-
3Name the metal ions. The way you write the name will vary based on whether you’re using the newer or older naming method. So, to name the metal ion, either write Iron (III) or ferric.
-
4Jot down the name of the non-metal ions. Refer to the periodic table to remind yourself that “S” is sulfur. Ammonium is not an element, but occurs when 1 nitrogen ion combines with 4 hydrogen ions. So, you're working with ammonium and sulfate, or ammonium sulfate.
- “Ammonia” becomes “ammonium” when it takes on a positive charge. Ammonia itself is neutrally charged.
-
5Combine the name of the metal with the names of the non-metals. You can name the compound FeNH4(SO4)2 by writing iron (III) ammonium sulfate.[16]
- If you’re required to use the older naming method for ionic compounds, write ferric ammonium sulfate.
Expert Q&A
Did you know you can get expert answers for this article?
Unlock expert answers by supporting wikiHow
-
QuestionWhat do you call this compound: NaBr?
 Bess Ruff, MABess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
Bess Ruff, MABess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.
Environmental Scientist
-
QuestionHow do I find the charge of the anion?
 Community AnswerIf you look at the group numbers of non-transition metals, they can tell you charges. For example, Chlorine (Cl) is in group 17 (7A). This tells me that there are 7 valence electrons in Cl & that it only needs 1 more electron to have a full valence shell, and therefore a charge of (-1). If you try that with Group 5,6,7 elements, you will find the pattern (-3, -2, -1) for their charges. It gets more complicated if you have polyatomic ions, since the charge determination involves some calculation. Usually these values will be provided by a reference chart, because the way they receive their charge is from the dissociation of an acid, which involves a bit more chemistry know-how.
Community AnswerIf you look at the group numbers of non-transition metals, they can tell you charges. For example, Chlorine (Cl) is in group 17 (7A). This tells me that there are 7 valence electrons in Cl & that it only needs 1 more electron to have a full valence shell, and therefore a charge of (-1). If you try that with Group 5,6,7 elements, you will find the pattern (-3, -2, -1) for their charges. It gets more complicated if you have polyatomic ions, since the charge determination involves some calculation. Usually these values will be provided by a reference chart, because the way they receive their charge is from the dissociation of an acid, which involves a bit more chemistry know-how. -
QuestionIs there any simple method to identify the compound by observing its ionic properties?
 Community Answer+2 state has suffix '-ous', and +3 has '-ic' (for the central atom eg. Iron).
Community Answer+2 state has suffix '-ous', and +3 has '-ic' (for the central atom eg. Iron).
References
- ↑ https://medium.com/countdown-education/4-steps-to-naming-compounds-in-chemistry-nomenclature-7525ed57bd13
- ↑ https://sciencing.com/how-to-name-ionic-compounds-13712152.html
- ↑ https://sciencing.com/how-to-name-ionic-compounds-13712152.html
- ↑ https://sciencing.com/how-to-name-ionic-compounds-13712152.html
- ↑ https://youtu.be/7Lfc6jjp1WQ?t=40s
- ↑ https://medium.com/countdown-education/4-steps-to-naming-compounds-in-chemistry-nomenclature-7525ed57bd13
- ↑ https://sciencing.com/how-to-name-ionic-compounds-13712152.html
- ↑ https://medium.com/countdown-education/4-steps-to-naming-compounds-in-chemistry-nomenclature-7525ed57bd13
- ↑ https://www.dummies.com/education/science/chemistry/how-ionic-compounds-are-named/
- ↑ https://youtu.be/7Lfc6jjp1WQ?t=2m41s
- ↑ https://www.dummies.com/education/science/chemistry/how-ionic-compounds-are-named/
- ↑ https://youtu.be/7Lfc6jjp1WQ?t=3m32s
- ↑ http://www.dummies.com/how-to/content/how-ionic-compounds-are-named.html
- ↑ https://www.dummies.com/education/science/chemistry/how-ionic-compounds-are-named/
- ↑ http://www.essentialchemicalindustry.org/chemicals/sulfuric-acid.html
- ↑ https://www.dummies.com/education/science/chemistry/how-ionic-compounds-are-named/
About This Article
If you need to name an ionic compound, start by writing down the formula for that compound. Write the name of the metal, also called the cation. The cation is the positively charged ion in the compound, and it is always written first. Next, write the name of the nonmetal, or the anion. Drop the last syllable of the anion and add “ide” instead. For help naming transition metals, read on!