This article was co-authored by wikiHow staff writer, Danielle Blinka, MA, MPA. Danielle Blinka is a Writer, Editor, Podcaster, Improv Performer, and Artist currently living in Houston, TX. She also has experience teaching English and writing to others. Danielle holds a Bachelor of Arts in English, Bachelor of Arts in Political Science, Master of Arts in English with a concentration in writing, and Master of Public Administration from Lamar University.
There are 9 references cited in this article, which can be found at the bottom of the page.
Learn more...
Nothing stings like hydrogen peroxide on a wound. At least it’s cleaning your wound, right? While that’s true, there’s a lot more going on than you may realize. We’re here to answer any (and all) questions you’ve ever had about hydrogen peroxide—and some of the answers might surprise you.
Things You Should Know
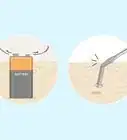
- Hydrogen peroxide bubbles when a chemical reaction breaks it down to water and oxygen.
- Organic materials like blood, damaged skin cells, and some bacteria contain an enzyme called catalase that sets off the reaction.
- Even though hydrogen peroxide cleans wounds, it’s not recommended for wound care because it irritates your skin and delays healing.
- Instead of using peroxide, wash your wound with soapy water, apply an ointment, and put on a bandage.
Steps
Warnings
- Never consume hydrogen peroxide because it can cause vomiting and stomach burns that may require medical care.[13]⧼thumbs_response⧽
References
- ↑ https://www.mcgill.ca/oss/article/general-science-you-asked/hydrogen-peroxide-bodys-best-defence-system
- ↑ https://www.sciencedirect.com/topics/earth-and-planetary-sciences/catalase
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/Hydrogen-peroxide
- ↑ https://www.nhs.uk/conditions/cuts-and-grazes/
- ↑ https://www.lvhn.org/sites/default/files/uploads/research_education/EmergencyMedicineMedStudents/WoundCleansing.pdf
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/Hydrogen-peroxide
- ↑ https://health.clevelandclinic.org/what-is-hydrogen-peroxide-good-for/
- ↑ https://www.houstonmethodist.org/blog/articles/2022/jun/should-you-put-hydrogen-peroxide-on-a-cut-or-scrape/
- ↑ https://health.clevelandclinic.org/what-is-hydrogen-peroxide-good-for/