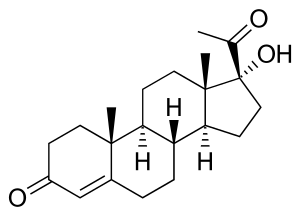
17α-Hydroxyprogesterone
17α-Hydroxyprogesterone (17α-OHP), also known as 17-OH progesterone (17-OHP),[1] or hydroxyprogesterone (OHP), is an endogenous progestogen steroid hormone related to progesterone.[2][3][4] It is also a chemical intermediate in the biosynthesis of many other endogenous steroids, including androgens, estrogens, glucocorticoids, and mineralocorticoids, as well as neurosteroids.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
17α-Hydroxypregn-4-ene-3,20-dione | |
| Systematic IUPAC name
(1R,3aS,3bR,9aR,9bS,11aS)-1-Acetyl-1-hydroxy-9a,11a-dimethyl-1,2,3,3a,3b,4,5,8,9,9a,9b,10,11,11a-tetradecahydro-7H-cyclopenta[a]phenanthren-7-one | |
| Other names
Hydroxyprogesterone (INNTooltip International Nonproprietary Name) | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.636 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H30O3 | |
| Molar mass | 330.46 g/mol |
| Melting point | 219.5 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Biological activity
17α-OHP is an agonist of the progesterone receptor (PR) similarly to progesterone, albeit weakly in comparison.[5] In addition, it is an antagonist of the mineralocorticoid receptor (MR)[6] as well as a partial agonist of the glucocorticoid receptor (GR), albeit with very low potency (EC50 >100-fold less relative to cortisol) at the latter site, also similarly to progesterone.[5][7][8]
| Compound | hPR-A | hPR-B | rbPR | rbGR | rbER | |||
|---|---|---|---|---|---|---|---|---|
| Progesterone | 100 | 100 | 100 | <1 | <1 | |||
| 17α-Hydroxyprogesterone | 1 | 1 | 3 | 1 | <1 | |||
| Hydroxyprogesterone caproate | 26 | 30 | 28 | 4 | <1 | |||
| Hydroxyprogesterone acetate | 38 | 46 | 115 | 3 | ? | |||
| Notes: Values are percentages (%). Reference ligands (100%) were progesterone for the PRTooltip progesterone receptor, dexamethasone for the GRTooltip glucocorticoid receptor, and estradiol for the ERTooltip estrogen receptor. Sources: See template. | ||||||||
Biochemistry
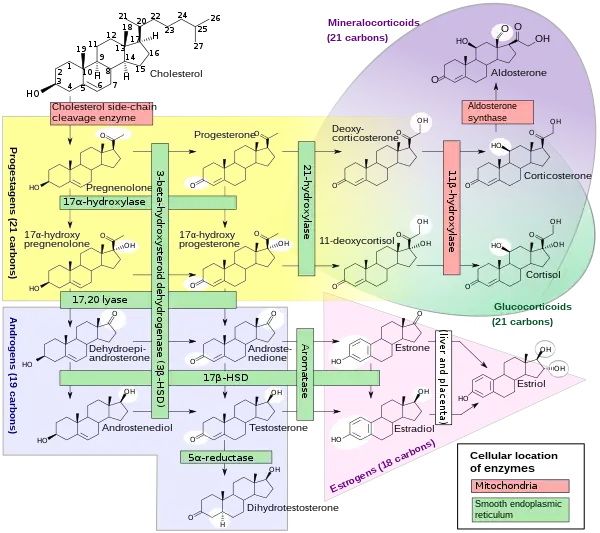
Biosynthesis
17α-OHP is derived from progesterone via 17α-hydroxylase (encoded by CYP17A1)
17α-OHP increases in the third trimester of pregnancy primarily due to fetal adrenal production.
This steroid is primarily produced in the adrenal glands and to some degree in the gonads, specifically the corpus luteum of the ovary. Normal levels are 3-90 ng/dl in children, and in women, 20-100 ng/dl prior to ovulation, and 100-500 ng/dl during the luteal phase.[9][10]
Measurement
Measurements of levels of 17α-OHP are useful in the evaluation of patients with suspected congenital adrenal hyperplasia as the typical enzymes that are defective, namely 21-hydroxylase and 11β-hydroxylase, lead to a build-up of 17α-OHP. In contrast, the rare patient with 17α-hydroxylase deficiency will have very low or undetectable levels of 17α-OHP. 17α-OHP levels can also be used to measure contribution of progestational activity of the corpus luteum during pregnancy as progesterone but note, 17α-OHP is also contributed by the placenta.
Immunoassays like RIA (radioimmunoassay) or IRMA (immunoradiometric assay) used to clinically determine 17α-OHP are prone to cross-reactivity with the 17α-OHP steroid precursors and their sulphated conjugates. Gas or liquid chromatography and mass spectrometry (e.g. LC-MS/MS) achieves greater specificity than immunoassays.[11][12]
Measurement of 17α-OHP by LC-MS/MS improves newborn screening for congenital adrenal hyperplasia due to 21-hydroxylase deficiency, because 17α-OHP steroid precursors and their sulphated conjugates which are present in the first two days after birth and longer in pre-term neonates, cross-react in immunoassays with 17α-OHP, giving falsely high 17α-OHP levels.[11][12]
Pharmacology
Pharmacokinetics
Although 17α-OHP has not been used as a medication, its pharmacokinetics have been studied and reviewed.[13]
Medical uses
Esters of 17α-OHP, such as hydroxyprogesterone caproate and, to a far lesser extent, hydroxyprogesterone acetate and hydroxyprogesterone heptanoate, have been used in medicine as progestins.[2][3][4] When "hydroxyprogesterone" is referenced from the standpoint of medical use, what is usually being referred to is actually, in general, hydroxyprogesterone caproate.
Chemistry
17α-OHP, also known as 17α-hydroxypregn-4-ene-3,20-dione, is a naturally occurring pregnane steroid. It features ketone groups at the C3 and C20 positions, a hydroxyl group at the C17α position, and a double bond between the C4 and C5 positions.
17α-OHP is the parent compound of a class of progestins referred to as the 17α-hydroxyprogesterone derivatives.[14][15][16] Among others, this class of drugs includes chlormadinone acetate, cyproterone acetate, hydroxyprogesterone caproate, medroxyprogesterone acetate, and megestrol acetate.[14][15][16]
Society and culture
See also
References
- "17-hydroxyprogesterone (17OHP)".
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 664–665. ISBN 978-1-4757-2085-3.
- I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 146–. ISBN 978-94-011-4439-1.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 532–. ISBN 978-3-88763-075-1.
- Attardi BJ, Zeleznik A, Simhan H, Chiao JP, Mattison DR, Caritis SN (2007). "Comparison of progesterone and glucocorticoid receptor binding and stimulation of gene expression by progesterone, 17-alpha hydroxyprogesterone caproate, and related progestins". Am. J. Obstet. Gynecol. 197 (6): 599.e1–7. doi:10.1016/j.ajog.2007.05.024. PMC 2278032. PMID 18060946.
- Mooij CF, Parajes S, Pijnenburg-Kleizen KJ, Arlt W, Krone N, Claahsen-van der Grinten HL (April 2015). "Influence of 17-Hydroxyprogesterone, Progesterone and Sex Steroids on Mineralocorticoid Receptor Transactivation in Congenital Adrenal Hyperplasia" (PDF). Horm Res Paediatr. 83 (6): 414–421. doi:10.1159/000374112. PMID 25896481. S2CID 24727940.
- Pijnenburg-Kleizen KJ, Engels M, Mooij CF, Griffin A, Krone N, Span PN, van Herwaarden AE, Sweep FC, Claahsen-van der Grinten HL (2015). "Adrenal Steroid Metabolites Accumulating in Congenital Adrenal Hyperplasia lead to Transactivation of the Glucocorticoid Receptor". Endocrinology. 156 (10): 3504–3510. doi:10.1210/en.2015-1087. PMID 26207344.
- Sun, Kang; Lei, Kaiyu; Chen, Li; Georgiou, Ektoras X.; Sooranna, Suren R.; Khanjani, Shirin; Brosens, Jan J.; Bennett, Phillip R.; Johnson, Mark R. (2012). "Progesterone Acts via the Nuclear Glucocorticoid Receptor to Suppress IL-1β-Induced COX-2 Expression in Human Term Myometrial Cells". PLOS ONE. 7 (11): e50167. Bibcode:2012PLoSO...750167L. doi:10.1371/journal.pone.0050167. ISSN 1932-6203. PMC 3509141. PMID 23209664.
- Reference Values During Pregnancy
- "normal ranges for hormone tests in women". Archived from the original on 2020-11-08. Retrieved 2011-08-07.
- de Hora MR, Heather NL, Patel T, Bresnahan LG, Webster D, Hofman PL (March 2020). "Measurement of 17-Hydroxyprogesterone by LCMSMS Improves Newborn Screening for CAH Due to 21-Hydroxylase Deficiency in New Zealand". International Journal of Neonatal Screening. 6 (1): 6. doi:10.3390/ijns6010006. PMC 7422986. PMID 33073005.
- Bialk ER, Lasarev MR, Held PK (September 2019). "Wisconsin's Screening Algorithm for the Identification of Newborns with Congenital Adrenal Hyperplasia". International Journal of Neonatal Screening. 5 (3): 33. doi:10.3390/ijns5030033. PMC 7510207. PMID 33072992.
- Die Gestagene. Springer-Verlag. 27 November 2013. pp. 276–277. ISBN 978-3-642-99941-3.
- Jeffrey K. Aronson (21 February 2009). Meyler's Side Effects of Endocrine and Metabolic Drugs. Elsevier. pp. 289–. ISBN 978-0-08-093292-7.
- Robert Alan Prentky; Ann Wolbert Burgess (31 July 2000). Forensic Management of Sexual Offenders. Springer Science & Business Media. pp. 219–. ISBN 978-0-306-46278-8.
- H. J. Smith; Hywel Williams (1 January 1983). Introduction to the Principles of Drug Design. Elsevier. pp. 187–. ISBN 978-1-4831-8350-3.