17β-Hydroxysteroid dehydrogenase
17β-Hydroxysteroid dehydrogenases (17β-HSD, HSD17B) (EC 1.1.1.51), also 17-ketosteroid reductases (17-KSR), are a group of alcohol oxidoreductases which catalyze the reduction of 17-ketosteroids and the dehydrogenation of 17β-hydroxysteroids in steroidogenesis and steroid metabolism.[1][2][3][4][5] This includes interconversion of DHEA and androstenediol, androstenedione and testosterone, and estrone and estradiol.[6][7]
| 17β-Hydroxysteroid dehydrogenase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 1.1.1.51 | ||||||||
| CAS no. | 9015-81-0 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
The major reactions catalyzed by 17β-HSD (e.g., the conversion of androstenedione to testosterone) are in fact hydrogenation (reduction) rather than dehydrogenation (oxidation) reactions.
Reactions
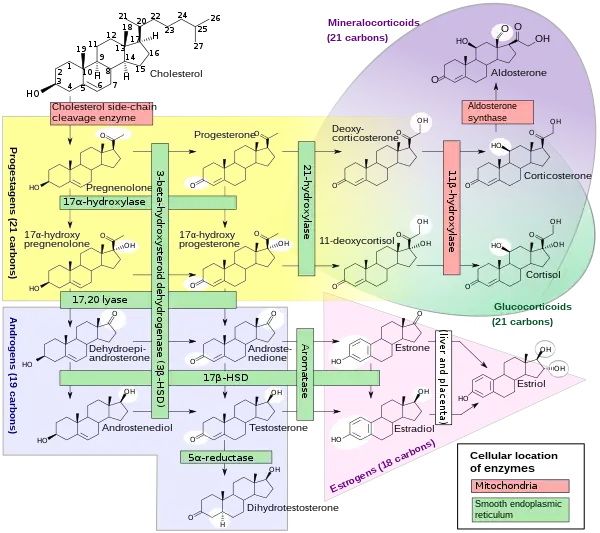
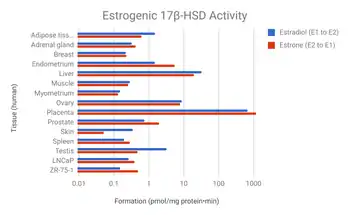
17β-HSDs have been known to catalyze the following redox reactions of sex steroids:
- 20α-Hydroxyprogesterone ↔ Progesterone
- DHEATooltip Dehydroepiandrosterone ↔ Androstenediol
- Androstenedione ↔ Testosterone
- Dihydrotestosterone ↔ 5α-Androstanedione / 3α-Androstanediol / 3β-Androstanediol
- Estrone ↔ Estradiol
- 16α-Hydroxyestrone ↔ Estriol
Genes
Genes coding for 17β-HSD include:
- HSD17B1: Referred to as "estrogenic". Major subtype for activation of estrogens from weaker forms (estrone to estradiol and 16α-hydroxyestrone to estriol). Catalyzes the final step in the biosynthesis of estrogens. Highly selective for estrogens; 100-fold higher affinity for estranes over androstanes. However, also catalyzes the conversion of DHEA into androstenediol.[10] Recently, has been found to inactivate DHT into 3α- and 3β-androstanediol.[10][11] Expressed primarily in the ovaries and placenta but also at lower levels in the breast epithelium.[12][10] Major isoform of 17β-HSD in the granulosa cells of the ovaries.[13] Mutations and associated deficiency have not been reported in humans.[14] Knockout mice show altered ovarian sex steroid production, normal puberty, and severe subfertility due to defective luteinization and ovarian progesterone production.[15]
- HSD17B2: Describable as "antiestrogenic" and "antiandrogenic".[16] Major subtype for inactivation of estrogens and androgens into weaker forms (estradiol to estrone, testosterone to androstenedione, and androstenediol to DHEA). Also converts inactive 20α-hydroxyprogesterone into active progesterone. Preferential activity on androgens. Expressed widely in the body including in the liver, intestines, lungs, pancreas, kidneys, endometrium, prostate, breast epithelium, placenta, and bone.[10][17][12] Said to be responsible for 17β-HSD activity in the endometrium and placenta.[18] Mutations and associated congenital deficiency have not been reported in humans.[14] However, local deficiency in expression of HSD17B2 has been associated with endometriosis.[19]
- HSD17B3: Referred to as "androgenic". Major subtype in males for activation of androgens from weaker forms (androstenedione to testosterone and DHEA to androstenediol). Also activates estrogens from weaker forms to a lesser extent (estrone to estradiol). This is essential for testicular but not ovarian production of testosterone. Not expressed in the ovaries, where another 17β-HSD subtype, likely HSD17B5, is expressed instead. Mutations are associated with 17β-HSD type III deficiency. Males with this condition have pseudohermaphroditism, while females are normal with normal androgen and estrogen levels.[17][12]
- HSD17B4: Also known as D-bifunctional protein (DBP). Involved in fatty acid β-oxidation and steroid metabolism (specifically estrone to estradiol, for instance in the uterus).[20] Mutations are associated with DBP deficiency and Perrault syndrome (ovarian dysgenesis and deafness).[20]
- HSD17B5: Also known as aldo-keto reductase 1C3 (AKR1C3). Has 3α-HSDTooltip 3α-hydroxysteroid dehydrogenase and 20α-HSDTooltip 20α-hydroxysteroid dehydrogenase activity in addition to 17β-HSD activity. Expressed in the adrenal cortex and may act as the "androgenic" 17β-HSD in ovarian thecal cells. Also expressed in the prostate gland, mammary gland, and Leydig cells.[12]
- HSD17B6: Has 3α-HSDTooltip 3α-hydroxysteroid dehydrogenase activity and catalyzes conversion of the weak androgen androstanediol into the powerful androgen dihydrotestosterone in the prostate gland. Also involved into a backdoor pathway from 17α-hydroxyprogesterone to dihydrotestosterone by 3α-reduction of a metabolic intermediary, 17α-hydroxydihydroprogesterone, into another intermediary, 17α-hydroxyallopregnanolone.[21] May be involved in the pathophysiology of PCOSTooltip polycystic ovary syndrome.[12]
- HSD17B7: Is involved in cholesterol metabolism but is also thought to activate estrogens (estrone to estradiol) and inactivate androgens (dihydrotestosterone to androstanediol).[12] Expressed in the ovaries, breasts, placenta, testes, prostate gland, and liver.[12]
- HSD17B8: Inactivates estradiol, testosterone, and dihydrotestosterone, though can also convert estrone into estradiol. Expressed in the ovaries, testes, liver, pancreas, kidneys, and other tissues.[22][23]
- HSD17B9: Also known as retinol dehydrogenase 5 (RDH5). Involved in retinoid metabolism.[24] Mutations are associated with fundus albipunctatus.[25]
- HSD17B10: Also known as 2-methyl-3-hydroxybutyryl-CoA dehydrogenase (MHBD). Substrates include steroids, neurosteroids, fatty acids, bile acids, isoleucine, and xenobiotics.[26][27] Mutations are associated with 17β-HSD type X deficiency (also known as HSD10 disease or MHBD deficiency) and mental retardation, X-linked, syndromic 10 (MRXS10), which are characterized by neurodegeneration and mental retardation, respectively.[26][27]
- HSD17B11
- HSD17B12
- HSD17B13
- HSD17B14
At least 7 of the 14 isoforms of 17β-HSD are involved in interconversion of 17-ketosteroids and 17β-hydroxysteroids.[12]
Overview
| # | Gene name | Synonyms | Family | Size (AATooltip Amino acids) | Gene location | Cellular location | Substrate specificities | Preferred cofactor | Catalytic preference | Tissue distribution | Expression profile | Pathology |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | HSD17B1 | SDRTooltip Short-chain dehydrogenase | 328 | 17q21.2 | Cytosol | Estrogens | NADH, NADPH | Reduction | Ovary, endometrium, breast, brain, prostate, placenta | Strongly restricted | Breast cancer, prostate cancer, endometriosis | |
| 2 | HSD17B2 | SDR | 387 | 16q23.3 | ERTooltip Endoplasmic reticulum | Estrogens, androgens, progestogens | NAD+ | Oxidation | Liver, intestine, endometrium, placenta, pancreas, prostate, colon, bone | Selectively distributed | Breast cancer, prostate cancer, endometriosis, osteoporosis[32] | |
| 3 | HSD17B3 | SDR | 310 | 9q22.32 | ER | Androgens | NADPH | Reduction | Testis, ovary, blood, saliva, skin, adipose tissue, brain, bone | Strongly restricted | 17β-HSD3 deficiency, prostate cancer[33] | |
| 4 | HSD17B4 | DBP, MFP2 | SDR | 736 | 5q23.1 | PXSTooltip Peroxisomes | Fatty acids, bile acids, estrogens, androgens | NAD+ | Oxidation | Liver, heart, prostate, testis, lung, skeletal muscle, kidney, pancreas, thymus, ovary, intestine, placenta, brain, spleen, colon, lymphocytes | Ubiquitous | DBP deficiency, Perrault syndrome, prostate cancer |
| 5 | AKR1C3Tooltip Aldo-keto reductase family 1 member C3 | HSD17B5, PGFS | AKRTooltip Aldo-keto reductase | 323 | 10p15.1 | Nucleus, cytosol | Androgens, progestogens, estrogens, prostaglandins | NADPH | Reduction | Prostate, mammary gland, liver, kidney, lung, heart, small intestine, colon, uterus, testis, brain, skeletal muscle, adipose tissue | Nearly ubiquitous | Breast cancer, prostate cancer |
| 6 | HSD17B6 | SDR | 317 | 12q13.3 | Endosomes | Retinoids, androgens, estrogens | NAD+ | Oxidation | Liver, testis, lung, spleen, brain, ovary, kidney, adrenal, prostate | Selectively distributed | ? | |
| 7 | HSD17B7 | SDR | 341 | 1q23.3 | PMTooltip Plasma membrane, ER | Cholesterol, estrogens, androgens, progestogens | NADPH | Reduction | Ovary, corpus luteum, uterus, placenta, liver, breast, testis, brain, adrenal gland, small intestine, lung, thymus, prostate, adipose tissue, others | Widely distributed | Breast cancer | |
| 8 | HSD17B8 | SDR | 261 | 6p21.32 | MCTooltip Mitochondria | Fatty acids, estrogens, androgens | NAD+ | Oxidation | Prostate, placenta, kidney, brain, cerebellum, heart, lung, small intestine, ovary, testis, adrenal, stomach | Widely distributed | Polycystic kidney disease | |
| 9 | RDH5Tooltip Retinol dehydrogenase 5 | HSD17B9 | 318 | 12q13.2 | ER | Retinoids | NADH/NAD+ | Reduction / oxidation | Retina, liver, adipose tissue, blood, others | ? | Fundus albipunctatus | |
| 10 | HSD17B10 | MHBD | SDR | 261 | Xp11.2 | MC | Fatty acids, bile acids, estrogens, androgens, progestogens, corticosteroids | NAD+ | Oxidation | Liver, small intestine, colon, kidney, heart, brain, placenta, lung, ovary, testis, spleen, thymus, prostate, peripheral blood leukocytes | Nearly ubiquitous | 17β-HSD10 deficiency, MRXS10Tooltip Mental retardation, X-linked, syndromic 10, Alzheimer's disease |
| 11 | HSD17B11 | SDR | 300 | 4q22.1 | ER, EPTooltip Extracellular space | Estrogens, androgens | NAD+ | Oxidation | Liver, pancreas, intestine, kidney, adrenal gland, heart, lung, testis, ovary, placenta, sebaceous gland | Nearly ubiquitous | ? | |
| 12 | HSD17B12 | SDR | 312 | 11p11.2 | ER | Fatty acids, estrogens, androgens | NADPH | Reduction | Heart, skeletal muscle, liver, kidney, adrenal gland, testis, placenta, cerebellum, pancreas, stomach, small intestine, large intestine, trachea, lung, thyroid, esophagus, prostate, aorta, urinary bladder, spleen, skin, brain, ovary, breast, uterus, vagina | Ubiquitous | ? | |
| 13 | HSD17B13 | SDR | 300 | 4q22.1 | ER, EP | ? | NAD+? | Oxidation? | Liver, bone marrow, lung, ovary, testis, kidney, skeletal muscle, brain, bladder, nasal epithelia | Strongly restricted | ? | |
| 14 | HSD17B14 | SDR | 270 | 19q13.33 | Cytosol | Estrogens, androgens, fatty acids | NAD+ | Oxidation | Liver, kidney, brain, gallbladder, breast, adrenal, placenta | Widely distributed | Breast cancer (prognostic) | |
| 15 | RDH11Tooltip Retinol dehydrogenase 11[34][35][36] | PSDR1, HSD17B15 | SDR | 318 | 14q23-24.3 | ER | Retinoids, androgens | NADPH | Reduction | Retina, prostate, brain, testis | ? | Retinitis pigmentosa[37] |
Clinical significance
Mutations in HSD17B3 are responsible for 17β-HSD type III deficiency.
Inhibitors of 17β-HSD type II are of interest for the potential treatment of osteoporosis.[32][38]
Some inhibitors of 17β-HSD type I have been identified, for example esters of cinnamic acid and various flavones (e.g. fisetin).[39]
See also
References
- Dahm K, Breuer H (1964). "Anreicherung einer 17β-hydroxysteroid:NAD(P)-oxydoreduktase aus der Nebenniere der Ratte" [Precipitation of a 17-Beta-Hydroxysteroid:Nad(P) Oxidoreductase from the Rat Adrenal Gland]. Hoppe-Seyler's Zeitschrift für Physiologische Chemie (in German). 336: 63–8. doi:10.1515/bchm2.1964.336.1.63. PMID 14214322.
- Lynn WS, Brown RH (June 1958). "The conversion of progesterone to androgens by testes". The Journal of Biological Chemistry. 232 (2): 1015–30. doi:10.1016/S0021-9258(19)77419-5. PMID 13549484.
- Marcus PI, Talalay P (February 1956). "Induction and purification of alpha- and beta-hydroxysteroid dehydrogenases". The Journal of Biological Chemistry. 218 (2): 661–74. doi:10.1016/S0021-9258(18)65833-8. PMID 13295221.
- Schultz RM, Groman EV, Engel LL (June 1977). "3(17)beta-Hydroxysteroid dehydrogenase of Pseudomonas testosteroni. A convenient purification and demonstration of multiple molecular forms". The Journal of Biological Chemistry. 252 (11): 3775–83. doi:10.1016/S0021-9258(17)40319-X. PMID 193845.
- Talalay P, Dobson MM (December 1953). "Purification and properties of a beta-hydroxysteroid dehydrogenase". The Journal of Biological Chemistry. 205 (2): 823–37. doi:10.1016/S0021-9258(18)49226-5. PMID 13129261.
- Labrie F, Luu-The V, Lin SX, Labrie C, Simard J, Breton R, Bélanger A (January 1997). "The key role of 17 beta-hydroxysteroid dehydrogenases in sex steroid biology". Steroids. 62 (1): 148–58. doi:10.1016/S0039-128X(96)00174-2. PMID 9029730. S2CID 54365519.
- Brook CG, Truong D, Clayton P, Carroll W, Brown R (2011). Brook's Clinical Pediatric Endocrinology. John Wiley & Sons. p. 288. ISBN 978-1-4443-1673-5.
- Martel C, Rhéaume E, Takahashi M, Trudel C, Couët J, Luu-The V, Simard J, Labrie F (March 1992). "Distribution of 17 beta-hydroxysteroid dehydrogenase gene expression and activity in rat and human tissues". J. Steroid Biochem. Mol. Biol. 41 (3–8): 597–603. doi:10.1016/0960-0760(92)90390-5. PMID 1314080. S2CID 54325300.
- Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. p. 226. ISBN 978-3-642-58616-3.
- Hilborn E, Stål O, Jansson A (May 2017). "Estrogen and androgen-converting enzymes 17β-hydroxysteroid dehydrogenase and their involvement in cancer: with a special focus on 17β-hydroxysteroid dehydrogenase type 1, 2, and breast cancer". Oncotarget. 8 (18): 30552–30562. doi:10.18632/oncotarget.15547. PMC 5444764. PMID 28430630.
- Aka JA, Mazumdar M, Chen CQ, Poirier D, Lin SX (April 2010). "17beta-hydroxysteroid dehydrogenase type 1 stimulates breast cancer by dihydrotestosterone inactivation in addition to estradiol production". Molecular Endocrinology. 24 (4): 832–45. doi:10.1210/me.2009-0468. PMC 5417535. PMID 20172961.
- Strauss JF, Barbieri RL (13 September 2013). Yen and Jaffe's Reproductive Endocrinology. Elsevier Health Sciences. p. 82. ISBN 978-1-4557-2758-2.
- Andersson S, Moghrabi N (January 1997). "Physiology and molecular genetics of 17 beta-hydroxysteroid dehydrogenases". Steroids. 62 (1): 143–7. doi:10.1016/s0039-128x(96)00173-0. PMID 9029729. S2CID 54341481.
- Jameson JL (13 July 1998). Principles of Molecular Medicine. Springer Science & Business Media. p. 549. ISBN 978-1-59259-726-0.
- Hakkarainen J, Jokela H, Pakarinen P, Heikelä H, Kätkänaho L, Vandenput L, Ohlsson C, Zhang FP, Poutanen M (September 2015). "Hydroxysteroid (17β)-dehydrogenase 1-deficient female mice present with normal puberty onset but are severely subfertile due to a defect in luteinization and progesterone production". FASEB Journal. 29 (9): 3806–16. doi:10.1096/fj.14-269035. PMID 26018678.
- Wang CT, Li CF, Wu WJ, Huang CN, Li CC, Li WM, Chan TC, Liang PI, Hsing CH, Liao KM (2016). "High Expression of 17β-hydroxysteroid Dehydrogenase Type 2 is Associated with a Better Prognosis in Urothelial Carcinoma of the Urinary Tract". Journal of Cancer. 7 (15): 2221–2230. doi:10.7150/jca.16777. PMC 5166531. PMID 27994658.
HSD17B2 has both anti-estrogenic and anti-androgenic functions.
- Melmed S (2016). Williams Textbook of Endocrinology. Elsevier Health Sciences. p. 904. ISBN 978-0-323-29738-7.
- Jameson JL, De Groot LJ (25 February 2015). Endocrinology: Adult and Pediatric E-Book. Elsevier Health Sciences. p. 2078. ISBN 978-0-323-32195-2.
- Bulun SE, Cheng YH, Pavone ME, Yin P, Imir G, Utsunomiya H, Thung S, Xue Q, Marsh EE, Tokunaga H, Ishikawa H, Kurita T, Su EJ (January 2010). "17Beta-hydroxysteroid dehydrogenase-2 deficiency and progesterone resistance in endometriosis". Seminars in Reproductive Medicine. 28 (1): 44–50. doi:10.1055/s-0029-1242992. PMC 4511594. PMID 20108182.
- Pierce SB, Walsh T, Chisholm KM, Lee MK, Thornton AM, Fiumara A, Opitz JM, Levy-Lahad E, Klevit RE, King MC (August 2010). "Mutations in the DBP-deficiency protein HSD17B4 cause ovarian dysgenesis, hearing loss, and ataxia of Perrault Syndrome". American Journal of Human Genetics. 87 (2): 282–8. doi:10.1016/j.ajhg.2010.07.007. PMC 2917704. PMID 20673864.
- Masiutin, Maxim; Yadav, Maneesh (2023). "Alternative androgen pathways". WikiJournal of Medicine. 10: X. doi:10.15347/WJM/2023.003. S2CID 257943362.
- Fomitcheva J, Baker ME, Anderson E, Lee GY, Aziz N (August 1998). "Characterization of Ke 6, a new 17beta-hydroxysteroid dehydrogenase, and its expression in gonadal tissues". The Journal of Biological Chemistry. 273 (35): 22664–71. doi:10.1074/jbc.273.35.22664. PMID 9712896.
- Kikuti YY, Tamiya G, Ando A, Chen L, Kimura M, Ferreira E, Tsuji K, Trowsdale J, Inoko H (June 1997). "Physical mapping 220 kb centromeric of the human MHC and DNA sequence analysis of the 43-kb segment including the RING1, HKE6, and HKE4 genes". Genomics. 42 (3): 422–35. doi:10.1006/geno.1997.4745. PMID 9205114.
- Lidén M, Tryggvason K, Eriksson U (December 2003). "Structure and function of retinol dehydrogenases of the short chain dehydrogenase/reductase family". Molecular Aspects of Medicine. 24 (6): 403–9. doi:10.1016/s0098-2997(03)00036-0. PMID 14585311.
- Skorczyk-Werner A, Pawłowski P, Michalczuk M, Warowicka A, Wawrocka A, Wicher K, Bakunowicz-Łazarczyk A, Krawczyński MR (August 2015). "Fundus albipunctatus: review of the literature and report of a novel RDH5 gene mutation affecting the invariant tyrosine (p.Tyr175Phe)". Journal of Applied Genetics. 56 (3): 317–27. doi:10.1007/s13353-015-0281-x. PMC 4543405. PMID 25820994.
- Yang SY, He XY, Miller D (2011). "Hydroxysteroid (17β) dehydrogenase X in human health and disease". Mol. Cell. Endocrinol. 343 (1–2): 1–6. doi:10.1016/j.mce.2011.06.011. PMID 21708223. S2CID 8608312.
- Yang SY, He XY, Isaacs C, Dobkin C, Miller D, Philipp M (2014). "Roles of 17β-hydroxysteroid dehydrogenase type 10 in neurodegenerative disorders". J. Steroid Biochem. Mol. Biol. 143: 460–72. doi:10.1016/j.jsbmb.2014.07.001. PMID 25007702.
- Zhu Y, Imperato-McGinley JL (9 November 2016). "4.02: Disorders of Sexual Development in Males: Molecular Genetics, Epigenetics, Gender Identity, and Cognition". In Lightman S (ed.). Hormones, Brain and Behavior. Vol. 4: Clinical Important Effects of Hormones on Brain and Behavior. Elsevier Science. p. 69. ISBN 978-0-12-803608-2.
- Moeller G, Adamski J (2009). "Integrated view on 17beta-hydroxysteroid dehydrogenases". Mol. Cell. Endocrinol. 301 (1–2): 7–19. doi:10.1016/j.mce.2008.10.040. PMID 19027824. S2CID 30321495.
- Mindnich R, Möller G, Adamski J (2004). "The role of 17 beta-hydroxysteroid dehydrogenases". Mol. Cell. Endocrinol. 218 (1–2): 7–20. doi:10.1016/j.mce.2003.12.006. PMID 15130507. S2CID 26877571.
- Marchais-Oberwinkler S, Henn C, Möller G, Klein T, Negri M, Oster A, Spadaro A, Werth R, Wetzel M, Xu K, Frotscher M, Hartmann RW, Adamski J (2011). "17β-Hydroxysteroid dehydrogenases (17β-HSDs) as therapeutic targets: protein structures, functions, and recent progress in inhibitor development". J. Steroid Biochem. Mol. Biol. 125 (1–2): 66–82. doi:10.1016/j.jsbmb.2010.12.013. PMID 21193039. S2CID 23767100.
- Soubhye J, Alard IC, van Antwerpen P, Dufrasne F (2015). "Type 2 17-β hydroxysteroid dehydrogenase as a novel target for the treatment of osteoporosis". Future Med Chem. 7 (11): 1431–56. doi:10.4155/fmc.15.74. PMID 26230882.
- Ning X, Yang Y, Deng H, Zhang Q, Huang Y, Su Z, Fu Y, Xiang Q, Zhang S (2017). "Development of 17β-hydroxysteroid dehydrogenase type 3 as a target in hormone-dependent prostate cancer therapy". Steroids. 121: 10–16. doi:10.1016/j.steroids.2017.02.003. PMID 28267564. S2CID 32062736.
- Samson M, Labrie F, and Luu-The V (23 June 2012). "Characterization of Type 15 17β-Hydroxysteroid Dehydrogenase". Steroid Hormone Biosynthesis & Metabolism (Translational).
- Lin B, White JT, Ferguson C, Wang S, Vessella R, Bumgarner R, True LD, Hood L, Nelson PS (2001). "Prostate short-chain dehydrogenase reductase 1 (PSDR1): a new member of the short-chain steroid dehydrogenase/reductase family highly expressed in normal and neoplastic prostate epithelium". Cancer Res. 61 (4): 1611–8. PMID 11245473.
- Kedishvili NY, Chumakova OV, Chetyrkin SV, Belyaeva OV, Lapshina EA, Lin DW, Matsumura M, Nelson PS (2002). "Evidence that the human gene for prostate short-chain dehydrogenase/reductase (PSDR1) encodes a novel retinal reductase (RalR1)". J. Biol. Chem. 277 (32): 28909–15. doi:10.1074/jbc.M202588200. PMID 12036956.
- Xie YA, Lee W, Cai C, Gambin T, Nõupuu K, Sujirakul T, Ayuso C, Jhangiani S, Muzny D, Boerwinkle E, Gibbs R, Greenstein VC, Lupski JR, Tsang SH, Allikmets R (2014). "New syndrome with retinitis pigmentosa is caused by nonsense mutations in retinol dehydrogenase RDH11". Hum. Mol. Genet. 23 (21): 5774–80. doi:10.1093/hmg/ddu291. PMC 4189905. PMID 24916380.
- Perspicace E, Cozzoli L, Gargano EM, Hanke N, Carotti A, Hartmann RW, Marchais-Oberwinkler S (August 2014). "Novel, potent and selective 17β-hydroxysteroid dehydrogenase type 2 inhibitors as potential therapeutics for osteoporosis with dual human and mouse activities". European Journal of Medicinal Chemistry. 83: 317–37. doi:10.1016/j.ejmech.2014.06.036. PMID 24974351.
- Brozic P, Kocbek P, Sova M, Kristl J, Martens S, Adamski J, Gobec S, Lanisnik Rizner T (March 2009). "Flavonoids and cinnamic acid derivatives as inhibitors of 17beta-hydroxysteroid dehydrogenase type 1". Molecular and Cellular Endocrinology. 301 (1–2): 229–34. doi:10.1016/j.mce.2008.09.004. PMID 18835421. S2CID 26950431.
External links
- 3(or+17)beta-hydroxysteroid+dehydrogenase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)