20β-Dihydroprogesterone
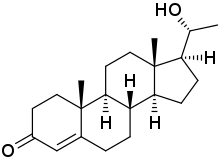
20β-Dihydroprogesterone (20β-DHP), also known as 20β-hydroxyprogesterone (20β-OHP), is an endogenous metabolite of progesterone which is formed by 20β-hydroxysteroid dehydrogenase (20β-HSD).[1] It is a progestogen similarly to progesterone, with about 20 to 50% of the progestogenic activity of progesterone.[1][2] It can be converted by 20β-HSD into progesterone in the uterus.[3] The effects of 20β-HSD on the uterus, mammary glands, and in maintaining pregnancy have been studied.[3][4][5] The progestogenic activity of 20β-HSD has also been characterized in women.[6][4]
 | |
| Names | |
|---|---|
| IUPAC name
(20R)-20-Hydroxypregn-4-en-3-one | |
| Systematic IUPAC name
(1S,3aS,3bS,9aR,9bS,11aS)-1-[(1R)-1-Hydroxyethyl]-9a,11a-dimethyl-1,2,3,3a,3b,4,5,8,9,9a,9b,10,11,11a-tetradecahydro-7H-cyclopenta[a]phenanthren-7-one | |
| Other names
20β-DHP; 20β-Hydroxyprogesterone; 20β-OHP; 20β-Progesterol; 20β-Progerol; 20β-Hydroxypregn-4-en-3-one; Pregn-4-en-20β-ol-3-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.137 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C21H32O2 | |
| Molar mass | 316.485 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
See also
References
- Bertram G. Katzung (30 November 2017). Basic and Clinical Pharmacology 14th Edition. McGraw-Hill Education. p. 728. ISBN 978-1-259-64116-9.
In addition to progesterone, 20α- and 20β-hydroxyprogesterone (20α- and 20β-hydroxy-4-pregnene-3-one) also are found. These compounds have about one-fifth the progestational activity of progesterone in humans and other species.
- Zander J, Forbes TR, Von Munstermann AM, Neher R (April 1958). "Delta 4-3-Ketopregnene-20 alpha-ol and delta 4-3-ketopregnene-20 beta-ol, two naturally occurring metabolites of progesterone; isolation, identification, biologic activity and concentration in human tissues". J. Clin. Endocrinol. Metab. 18 (4): 337–53. doi:10.1210/jcem-18-4-337. PMID 13513735.
- Lisboa, B. P.; Holtermann, M. (1976). "METABOLISM OF 20β-HYDROXY-4-PREGNEN-3-ONE IN UTERINE TISSUE OF NON-PREGNANT RATS IN VITRO". Acta Endocrinologica. 83 (3): 604–620. doi:10.1530/acta.0.0830604. ISSN 0804-4643. PMID 989999.
- Lauritzen, Christian (1963). "BIOLOGISCHE WIRKUNGEN DES 20β-HYDROXY-PREGN-4-EN-3-ON". Acta Endocrinologica. 44 (2): 225–236. doi:10.1530/acta.0.0440225. ISSN 0804-4643.
- Kumaresan, P.; Turner, C. W. (1968). "A Comparison of 20 -hydroxy-pregn-4-ene-3-one and Progesterone on Mammary Gland Growth of the Rat". Experimental Biology and Medicine. 129 (3): 955–956. doi:10.3181/00379727-129-33466. ISSN 1535-3702. PMID 5725127. S2CID 29178686.
- Besch, Paige K.; Barry, Roger D.; Vorys, Nichols; Stevens, Vernon; Ullery, John C. (1965). "A review of some aspects of the metabolism of progestational agents". Metabolism. 14 (3): 432–443. doi:10.1016/0026-0495(65)90031-4. ISSN 0026-0495. PMID 14261429.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.