Bacillus subtilis
Bacillus subtilis, known also as the hay bacillus or grass bacillus, is a Gram-positive, catalase-positive bacterium, found in soil and the gastrointestinal tract of ruminants, humans and marine sponges.[3][4][5][6] As a member of the genus Bacillus, B. subtilis is rod-shaped, and can form a tough, protective endospore, allowing it to tolerate extreme environmental conditions. B. subtilis has historically been classified as an obligate aerobe, though evidence exists that it is a facultative anaerobe. B. subtilis is considered the best studied Gram-positive bacterium and a model organism to study bacterial chromosome replication and cell differentiation. It is one of the bacterial champions in secreted enzyme production and used on an industrial scale by biotechnology companies.[3][4][5]
| Bacillus subtilis | |
|---|---|
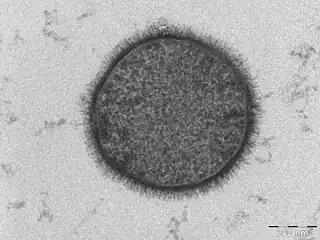 | |
| TEM micrograph of a B. subtilis cell in cross-section (scale bar = 200 nm) | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Bacillota |
| Class: | Bacilli |
| Order: | Bacillales |
| Family: | Bacillaceae |
| Genus: | Bacillus |
| Species: | B. subtilis |
| Binomial name | |
| Bacillus subtilis (Ehrenberg 1835) Cohn 1872 | |
| Synonyms | |
| |
Description
Bacillus subtilis is a Gram-positive bacterium, rod-shaped and catalase-positive. It was originally named Vibrio subtilis by Christian Gottfried Ehrenberg,[7] and renamed Bacillus subtilis by Ferdinand Cohn in 1872[8] (subtilis being the Latin for "fine, thin, slender"). B. subtilis cells are typically rod-shaped, and are about 4–10 micrometers (μm) long and 0.25–1.0 μm in diameter, with a cell volume of about 4.6 fL at stationary phase.[4][9]
As with other members of the genus Bacillus, it can form an endospore, to survive extreme environmental conditions of temperature and desiccation.[10] B. subtilis is a facultative anaerobe[4][11] and had been considered as an obligate aerobe until 1998. B. subtilis is heavily flagellated, which gives it the ability to move quickly in liquids.
B. subtilis has proven highly amenable to genetic manipulation, and has become widely adopted as a model organism for laboratory studies, especially of sporulation, which is a simplified example of cellular differentiation. In terms of popularity as a laboratory model organism, B. subtilis is often considered as the Gram-positive equivalent of Escherichia coli, an extensively studied Gram-negative bacterium.[12]
Characteristics
Colony, morphological, physiological, and biochemical characteristics of Bacillus subtilis are shown in the Table below.[4]
| Test type | Test | Characteristics |
| Colony characters | Size | Medium |
| Type | Round | |
| Color | Whitish | |
| Shape | Convex | |
| Morphological characters | Shape | Rod |
| Physiological characters | Motility | + |
| Growth at 6.5% NaCl | + | |
| Biochemical characters | Gram staining | + |
| Oxidase | - | |
| Catalase | + | |
| Oxidative-Fermentative | Fermentative | |
| Motility | - | |
| Methyl Red | - | |
| Voges-Proskauer | + | |
| Indole | - | |
| H2S Production | + | |
| Urease | - | |
| Nitrate reductase | + | |
| β-Galactosidase | + | |
| Hydrolysis of | Gelatin | + |
| Aesculin | + | |
| Casein | + | |
| Tween 40 | + | |
| Tween 60 | + | |
| Tween 80 | + | |
| Acid production from | Glycerol | + |
| Galactose | + | |
| D-Glucose | + | |
| D-Fructose | + | |
| D-Mannose | + | |
| Mannitol | + | |
| N-Acetylglucosamine | + | |
| Amygdalin | + | |
| Maltose | + | |
| D-Melibiose | + | |
| D-Trehalose | + | |
| Glycogen | + | |
| D-Turanose | + |
Note: + = Positive, – =Negative
Habitat
This species is commonly found in the upper layers of the soil and B. subtilis is thought to be a normal gut commensal in humans. A 2009 study compared the density of spores found in soil (about 106 spores per gram) to that found in human feces (about 104 spores per gram). The number of spores found in the human gut was too high to be attributed solely to consumption through food contamination.[13] In some bee habitats, B. subtilis appears in the gut flora of honey bees.[14] B. subtilis can also be found in marine environments.[4][5]
There is evidence that B. subtilis is saprophytic in nature. Studies have shown that the bacterium exhibits vegetative growth in soil rich in organic matter, and that spores were formed when nutrients were depleted.[15] Additionally, B. subtilis has been shown to form biofilms on plant roots, which might explain why it is commonly found in gut microbiomes.[15] Perhaps animals eating plants with B. subtilis biofilms can foster growth of the bacterium in their gastrointestinal tract. It has been shown that the entire lifecycle of B. subtilis can be completed in the gastrointestinal tract, which provides credence to the idea that the bacterium enters the gut via plant consumption and stays present as a result of its ability to grow in the gut.[15]
Reproduction

Bacillus subtilis can divide symmetrically to make two daughter cells (binary fission), or asymmetrically, producing a single endospore that can remain viable for decades and is resistant to unfavourable environmental conditions such as drought, salinity, extreme pH, radiation, and solvents. The endospore is formed at times of nutritional stress and through the use of hydrolysis, allowing the organism to persist in the environment until conditions become favourable. Prior to the process of sporulation the cells might become motile by producing flagella, take up DNA from the environment, or produce antibiotics.[4][5] These responses are viewed as attempts to seek out nutrients by seeking a more favourable environment, enabling the cell to make use of new beneficial genetic material or simply by killing off competition.
Under stressful conditions, such as nutrient deprivation, B. subtilis undergoes the process of sporulation. This process has been very well studied and has served as a model organism for studying sporulation.[16]
Sporulation
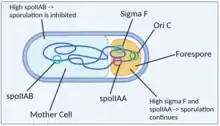
Once B. subtilis commits to sporulation, the sigma factor sigma F is secreted.[18] This factor promotes sporulation. A sporulation septum is formed and a chromosome is slowly moved into the forespore. When a third of one chromosome copy is in the forespore and the remaining two thirds is in the mother cell, the chromosome fragment in the forespore contains the locus for sigma F, which begins to be expressed in the forespore.[19] In order to prevent sigma F expression in the mother cell, an anti-sigma factor, which is encoded by spoIIAB,[20] is expressed. Any residual anti-sigma factor in the forespore (which would otherwise interfere with sporulation) is inhibited by an anti-anti-sigma factor, which is encoded by spoIIAA.[20] SpoIIAA is located near the locus for the sigma factor, so it is consistently expressed in the forespore. Since the spoIIAB locus is not located near the sigma F and spoIIAA loci, it is expressed only in the mother cell and therefore repress sporulation in that cell, allowing sporulation to continue in the forespore. Residual spoIIAA in the mother cell represses spoIIAB, but spoIIAB is constantly replaced so it continues to inhibit sporulation. When the full chromosome localizes to the forespore, spoIIAB can repress sigma F. Therefore, the genetic asymmetry of the B. subtilis chromosome and expression of sigma F, spoIIAB and spoIIAA dictate spore formation in B. subtilis.
Chromosomal replication
Bacillus subtilis is a model organism used to study bacterial chromosome replication. Replication of the single circular chromosome initiates at a single locus, the origin (oriC). Replication proceeds bidirectionally and two replication forks progress in clockwise and counterclockwise directions along the chromosome. Chromosome replication is completed when the forks reach the terminus region, which is positioned opposite to the origin on the chromosome map. The terminus region contains several short DNA sequences (Ter sites) that promote replication arrest. Specific proteins mediate all the steps in DNA replication. Comparison between the proteins involved in chromosomal DNA replication in B. subtilis and in Escherichia coli reveals similarities and differences. Although the basic components promoting initiation, elongation, and termination of replication are well-conserved, some important differences can be found (such as one bacterium missing proteins essential in the other). These differences underline the diversity in the mechanisms and strategies that various bacterial species have adopted to carry out the duplication of their genomes.[23]
Genome
Bacillus subtilis has about 4,100 genes. Of these, only 192 were shown to be indispensable; another 79 were predicted to be essential, as well. A vast majority of essential genes were categorized in relatively few domains of cell metabolism, with about half involved in information processing, one-fifth involved in the synthesis of cell envelope and the determination of cell shape and division, and one-tenth related to cell energetics.[24]
The complete genome sequence of B. subtilis sub-strain QB928 has 4,146,839 DNA base pairs and 4,292 genes. The QB928 strain is widely used in genetic studies due to the presence of various markers [aroI(aroK)906 purE1 dal(alrA)1 trpC2].[25]
Several noncoding RNAs have been characterized in the B. subtilis genome in 2009, including Bsr RNAs.[26] Microarray-based comparative genomic analyses have revealed that B. subtilis members show considerable genomic diversity.[27]
FsrA is a small RNA found in Bacillus subtilis. It is an effector of the iron sparing response, and acts to down-regulate iron-containing proteins in times of poor iron bioavailability.[28][29]
A promising fish probiotic, Bacillus subtilis strain WS1A, that possesses antimicrobial activity against Aeromonas veronii and suppressed motile Aeromonas septicemia in Labeo rohita. The de novo assembly resulted in an estimated chromosome size of 4,148,460 bp, with 4,288 open reading frames.[4][5] B. subtilis strain WS1A genome contains many potential genes, such as those encoding proteins involved in the biosynthesis of riboflavin, vitamin B6, and amino acids (ilvD) and in carbon utilization (pta).[4][5]
Transformation
Natural bacterial transformation involves the transfer of DNA from one bacterium to another through the surrounding medium. In B. subtilis the length of transferred DNA is greater than 1,271 kb (more than 1 million bases).[30] The transferred DNA is likely double-stranded DNA and is often more than a third of the total chromosome length of 4,215 kb.[31] It appears that about 7–9% of the recipient cells take up an entire chromosome.[32]
In order for a recipient bacterium to bind, take up exogenous DNA from another bacterium of the same species and recombine it into its chromosome, it must enter a special physiological state called competence. Competence in B. subtilis is induced toward the end of logarithmic growth, especially under conditions of amino-acid limitation.[33] Under these stressful conditions of semistarvation, cells typically have just one copy of their chromosome and likely have increased DNA damage. To test whether transformation is an adaptive function for B. subtilis to repair its DNA damage, experiments were conducted using UV light as the damaging agent.[34][35][36] These experiments led to the conclusion that competence, with uptake of DNA, is specifically induced by DNA-damaging conditions, and that transformation functions as a process for recombinational repair of DNA damage.[37]
While the natural competent state is common within laboratory B. subtilis and field isolates, some industrially relevant strains, e.g. B. subtilis (natto), are reluctant to DNA uptake due to the presence of restriction modification systems that degrade exogenous DNA. B. subtilis (natto) mutants, which are defective in a type I restriction modification system endonuclease, are able to act as recipients of conjugative plasmids in mating experiments, paving the way for further genetic engineering of this particular B. subtilis strain.[38]
By adopting Green Chemistry in the use of less hazardous materials, while saving cost, researchers have been mimicking nature's methods of synthesizing chemicals that can be useful for the food and drug industry, by "piggybacking molecules on shorts strands of DNA" before they are zipped together during their complimentary base pairing between the two strands. Each strand will carry a particular molecule of interest that will undergo a specific chemical reaction simultaneously when the two corresponding strands of DNA pairs hold together like a zipper, allowing another molecule of interest, to react with one another in controlled and isolated reaction between those molecules being carried into these DNA complimentary attachments. By using this method with certain bacterias that naturally follow a process replication in a multi-step fashion, the researchers can simultaneously carry on the interactions of these added molecules to interact with enzymes and other molecules used for a secondary reaction by treating it like a capsule, which is similar to how the bacteria performs its own DNA replication processes.[39]
Uses
20th century

Cultures of B. subtilis were popular worldwide, before the introduction of antibiotics, as an immunostimulatory agent to aid treatment of gastrointestinal and urinary tract diseases. It was used throughout the 1950s as an alternative medicine, which upon digestion has been found to significantly stimulate broad-spectrum immune activity including activation of secretion of specific antibodies IgM, IgG and IgA[40] and release of CpG dinucleotides inducing interferon IFN-α/IFNγ producing activity of leukocytes and cytokines important in the development of cytotoxicity towards tumor cells.[41] It was marketed throughout America and Europe from 1946 as an immunostimulatory aid in the treatment of gut and urinary tract diseases such as Rotavirus and Shigellosis. In 1966, the U.S. Army dumped bacillus subtilis onto the grates of New York City subway stations for five days in order to observe people's reactions when coated by a strange dust.[42] Due to its ability to survive, it is thought to still be present there.[43]
The antibiotic bacitracin was first isolated from a variety of Bacillus licheniformis named "Tracy I"[44] in 1945, then considered part of the B. subtilis species. It is still commercially manufactured by growing the variety in a container of liquid growth medium. Over time, the bacteria synthesizes bacitracin and secretes the antibiotic into the medium. The bacitracin is then extracted from the medium using chemical processes.[45]
Since the 1960s B. subtilis has had a history as a test species in spaceflight experimentation. Its endospores can survive up to 6 years in space if coated by dust particles protecting it from solar UV rays.[46] It has been used as an extremophile survival indicator in outer space such as Exobiology Radiation Assembly,[47][48] EXOSTACK,[49][50] and EXPOSE orbital missions.[51][52][53]
Wild-type natural isolates of B. subtilis are difficult to work with compared to laboratory strains that have undergone domestication processes of mutagenesis and selection. These strains often have improved capabilities of transformation (uptake and integration of environmental DNA), growth, and loss of abilities needed "in the wild". And, while dozens of different strains fitting this description exist, the strain designated '168' is the most widely used. Strain 168 is a tryptophan auxotroph isolated after X-ray mutagenesis of B. subtilis Marburg strain and is widely used in research due to its high transformation efficiency.[54]

Bacillus globigii, a closely related but phylogenetically distinct species now known as Bacillus atrophaeus[55][56] was used as a biowarfare simulant during Project SHAD (aka Project 112).[57] Subsequent genomic analysis showed that the strains used in those studies were products of deliberate enrichment for strains that exhibited abnormally high rates of sporulation.[58]
A strain of B. subtilis formerly known as Bacillus natto is used in the commercial production of the Japanese food nattō, as well as the similar Korean food cheonggukjang.
21st century
- As a model organism, B. subtilis is commonly used in laboratory studies directed at discovering the fundamental properties and characteristics of Gram-positive spore-forming bacteria.[27] In particular, the basic principles and mechanisms underlying formation of the durable endospore have been deduced from studies of spore formation in B. subtilis.
- Its surface-binding properties play a role in safe radionuclide waste [e.g. thorium (IV) and plutonium (IV)] disposal.
- Due to its excellent fermentation properties, with high product yields (20 to 25 gram per litre) it is used to produce various enzymes, such as amylase and proteases.[59]
- B. subtilis is used as a soil inoculant in horticulture and agriculture.[60][61][62]
- It may provide some benefit to saffron growers by speeding corm growth and increasing stigma biomass yield.[63]
- It is used as an "indicator organism" during gas sterilization procedures, to ensure a sterilization cycle has completed successfully.[64][65] This is due to the difficulty in sterilizing endospores.
- B. subtilis has been found to act as a useful bioproduct fungicide that prevents the growth of Monilinia vaccinii-corymbosi, a.k.a. the mummy berry fungus, without interfering with pollination or fruit qualities.[66]
- Both metabolically active and non-metabolically active B. subtilis cells have been shown to reduce gold (III) to gold (I) and gold (0) when oxygen is present. This biotic reduction plays a role in gold cycling in geological systems and could potentially be used to recover solid gold from said systems.
Novel and artificial substrains
- Novel strains of B. subtilis that could use 4-fluorotryptophan (4FTrp) but not canonical tryptophan (Trp) for propagation were isolated. As Trp is only coded by a single codon, there is evidence that Trp can be displaced by 4FTrp in the genetic code. The experiments showed that the canonical genetic code can be mutable.[67]
- Recombinant strains pBE2C1 and pBE2C1AB were used in production of polyhydroxyalkanoates (PHA), and malt waste can be used as their carbon source for lower-cost PHA production.
- It is used to produce hyaluronic acid, which is used in the joint-care sector in healthcare[68] and cosmetics.
- Monsanto has isolated a gene from B. subtilis that expresses cold shock protein B and spliced it into their drought-tolerant corn hybrid MON 87460, which was approved for sale in the US in November 2011.[69][70]
- A new strain has been modified to convert nectar into honey by secreting enzymes.[71]
Safety
In other animals
Bacillus subtilis was reviewed by the US FDA Center for Veterinary Medicine and found to present no safety concerns when used in direct-fed microbial products, so the Association of American Feed Control Officials has listed it approved for use as an animal feed ingredient under Section 36.14 "Direct-fed Microorganisms". The Canadian Food Inspection Agency Animal Health and Production Feed Section has classified Bacillus culture dehydrated approved feed ingredients as a silage additive under Schedule IV-Part 2-Class 8.6 and assigned the International Feed Ingredient number IFN 8-19-119. On the other hand, several feed additives containing viable spores of B. subtilis have been positively evaluated by the European Food Safety Authority, regarding their safe use for weight gaining in animal production.
In humans
Bacillus subtilis spores can survive the extreme heat generated during cooking. Some B. subtilis strains are responsible for causing ropiness or rope spoilage – a sticky, stringy consistency caused by bacterial production of long-chain polysaccharides – in spoiled bread dough and baked goods.[72] For a long time, bread ropiness was associated uniquely with B. subtilis species by biochemical tests. Molecular assays (randomly amplified polymorphic DNA PCR assay, denaturing gradient gel electrophoresis analysis, and sequencing of the V3 region of 16S ribosomal DNA) revealed greater Bacillus species variety in ropy breads, which all seems to have a positive amylase activity and high heat resistance.[73]
B. subtilis CU1 (2 × 109 spores per day) was evaluated in a 16-week study (10 days administration of probiotic, followed by 18 days wash-out period per each month; repeated same procedure for total 4 months) to healthy subjects. B. subtilis CU1 was found to be safe and well tolerated in the subjects without any side effects.[74]
Bacillus subtilis and substances derived from it have been evaluated by different authoritative bodies for their safe and beneficial use in food. In the United States, an opinion letter issued in the early 1960s by the Food and Drug Administration (FDA) designated some substances derived from microorganisms as generally recognized as safe (GRAS), including carbohydrase and protease enzymes from B. subtilis. The opinions were predicated on the use of nonpathogenic and nontoxicogenic strains of the respective organisms and on the use of current good manufacturing practices.[75] The FDA stated that the enzymes derived from the B. subtilis strain were in common use in food prior to January 1, 1958, and that nontoxigenic and nonpathogenic strains of B. subtilis are widely available and have been safely used in a variety of food applications. This includes consumption of Japanese fermented soy bean, in the form of Natto, which is commonly consumed in Japan, and contains as many as 108 viable cells per gram. The fermented beans are recognized for their contribution to a healthy gut flora and vitamin K2 intake; during this long history of widespread use, natto has not been implicated in adverse events potentially attributable to the presence of B. subtilis. The natto product and the B. subtilis natto as its principal component are FOSHU (Foods for Specified Health Use) approved by the Japanese Ministry of Health, Labour, and Welfare as effective for preservation of health.[76]
Bacillus subtilis has been granted "Qualified Presumption of Safety" status by the European Food Safety Authority.[77]
References
- Euzéby JP (2008). "Bacillus". List of Prokaryotic names with Standing in Nomenclature. Retrieved 2008-11-18.
- Ambrosiano N (1999-06-30). "Lab biodetector tests to be safe, public to be well informed". Press release. Los Alamos National Labs. Archived from the original on September 21, 2008. Retrieved 2008-11-18.
- Errington J, Aart LT (May 2020). "Microbe Profile: Bacillus subtilis: model organism for cellular development, and industrial workhorse". Microbiology. 166 (5): 425–427. doi:10.1099/mic.0.000922. PMC 7376258. PMID 32391747.
- Paul SI, Rahman MM, Salam MA, Khan MA, Islam MT (2021-12-15). "Identification of marine sponge-associated bacteria of the Saint Martin's island of the Bay of Bengal emphasizing on the prevention of motile Aeromonas septicemia in Labeo rohita". Aquaculture. 545: 737156. doi:10.1016/j.aquaculture.2021.737156. ISSN 0044-8486.
- Rahman MM, Paul SI, Akter T, Tay AC, Foysal MJ, Islam MT (September 2020). "Whole-Genome Sequence of Bacillus subtilis WS1A, a Promising Fish Probiotic Strain Isolated from Marine Sponge of the Bay of Bengal". Microbiology Resource Announcements. 9 (39). doi:10.1128/mra.00641-20. PMC 7516141. PMID 32972930.
- Paul SI, Rahman MM (October 2022). Gill SR (ed.). "Draft Genome Sequence of Bacillus subtilis YBS29, a Potential Fish Probiotic That Prevents Motile Aeromonas Septicemia in Labeo rohita". Microbiology Resource Announcements. 11 (10): e0091522. doi:10.1128/mra.00915-22. PMC 9583808. PMID 36154193.
- Ehrenberg CG (1835). Physikalische Abhandlungen der Koeniglichen Akademie der Wissenschaften zu Berlin aus den Jahren 1833–1835. pp. 145–336.
- Cohn F (1872). "Untersuchungen über Bacterien". Beiträge zur Biologie der Pflanzen. Vol. 1. pp. 127–224.
- Yu AC, Loo JF, Yu S, Kong SK, Chan TF (January 2014). "Monitoring bacterial growth using tunable resistive pulse sensing with a pore-based technique". Applied Microbiology and Biotechnology. 98 (2): 855–62. doi:10.1007/s00253-013-5377-9. PMID 24287933. S2CID 2956197.
- Madigan M, Martinko J, eds. (2005). Brock Biology of Microorganisms (11th ed.). Prentice Hall. ISBN 978-0-13-144329-7.
- Nakano MM, Zuber P (1998). "Anaerobic growth of a "strict aerobe" (Bacillus subtilis)". Annual Review of Microbiology. 52 (1): 165–90. doi:10.1146/annurev.micro.52.1.165. PMID 9891797.
- Ruiz N, Silhavy TJ (September 2022). "How Escherichia coli Became the Flagship Bacterium of Molecular Biology". Journal of Bacteriology. 204 (9): e0023022. doi:10.1128/jb.00230-22. PMC 9487582. PMID 35916528. S2CID 251254431.
- Hong HA, Khaneja R, Tam NM, Cazzato A, Tan S, Urdaci M, Brisson A, Gasbarrini A, Barnes I, Cutting SM (March 2009). "Bacillus subtilis isolated from the human gastrointestinal tract". Research in Microbiology. 160 (2): 134–43. doi:10.1016/j.resmic.2008.11.002. PMID 19068230.
- Sudhagar S, Reddy PR, Nagalakshmi G (April 2017). "Influence of elevation in structuring the gut bacterial communities of Apis cerana Fab" (PDF). Journal of Entomology and Zoology. 5 (3): 434–440.
- Tan IS, Ramamurthi KS (June 2014). "Spore formation in B acillus subtilis: Bacillus subtilis sporulation". Environmental Microbiology Reports. 6 (3): 212–225. doi:10.1111/1758-2229.12130. PMC 4078662.
- McKenney PT, Driks A, Eichenberger P (January 2013). "The Bacillus subtilis endospore: assembly and functions of the multilayered coat". Nature Reviews. Microbiology. 11 (1): 33–44. doi:10.1038/nrmicro2921. PMC 9910062. PMID 23202530. S2CID 205498395.
- Stephens C (January 1998). "Bacterial sporulation: a question of commitment?". Current Biology. 8 (2): R45–R48. doi:10.1016/S0960-9822(98)70031-4. PMID 9427639. S2CID 14126998.
- Earl AM, Losick R, Kolter R (June 2008). "Ecology and genomics of Bacillus subtilis". Trends in Microbiology. 16 (6): 269–275. doi:10.1016/j.tim.2008.03.004. PMC 2819312. PMID 18467096.
- Higgins D, Dworkin J (January 2012). "Recent progress in Bacillus subtilis sporulation". FEMS Microbiology Reviews. 36 (1): 131–148. doi:10.1111/j.1574-6976.2011.00310.x. PMC 3237856. PMID 22091839.
- Slonczewski J, John Watkins Foster, Zinser ER. 2020. Microbiology : an evolving science. New York: W.W. Norton & Company.
- Piggot PJ, Coote JG (December 1976). "Genetic aspects of bacterial endospore formation". Bacteriological Reviews. 40 (4): 908–962. doi:10.1128/MMBR.40.4.908-962.1976. PMC 413989. PMID 12736.
- Jabbari S, Heap JT, King JR (January 2011). "Mathematical modelling of the sporulation-initiation network in Bacillus subtilis revealing the dual role of the putative quorum-sensing signal molecule PhrA" (PDF). Bulletin of Mathematical Biology. 73 (1): 181–211. doi:10.1007/s11538-010-9530-7. PMID 20238180. S2CID 9875633.
- Noirot P (2007). "Replication of the Bacillus subtilis chromosome". In Graumann P (ed.). Bacillus: Cellular and Molecular Biology. Caister Academic Press. ISBN 978-1-904455-12-7.
- Kobayashi K, Ehrlich SD, Albertini A, Amati G, Andersen KK, Arnaud M, et al. (April 2003). "Essential Bacillus subtilis genes". Proceedings of the National Academy of Sciences of the United States of America. 100 (8): 4678–83. Bibcode:2003PNAS..100.4678K. doi:10.1073/pnas.0730515100. JSTOR 3144001. PMC 153615. PMID 12682299.
- Yu CS, Yim KY, Tsui SK, Chan TF (November 2012). "Complete genome sequence of Bacillus subtilis strain QB928, a strain widely used in B. subtilis genetic studies". Journal of Bacteriology. 194 (22): 6308–9. doi:10.1128/JB.01533-12. PMC 3486399. PMID 23105055.
- Saito S, Kakeshita H, Nakamura K (January 2009). "Novel small RNA-encoding genes in the intergenic regions of Bacillus subtilis". Gene. 428 (1–2): 2–8. doi:10.1016/j.gene.2008.09.024. PMID 18948176.
- Earl AM, Losick R, Kolter R (June 2008). "Ecology and genomics of Bacillus subtilis". Trends in Microbiology. 16 (6): 269–75. doi:10.1016/j.tim.2008.03.004. PMC 2819312. PMID 18467096.
- Gaballa A, Antelmann H, Aguilar C, Khakh SK, Song KB, Smaldone GT, Helmann JD (August 2008). "The Bacillus subtilis iron-sparing response is mediated by a Fur-regulated small RNA and three small, basic proteins". Proceedings of the National Academy of Sciences of the United States of America. 105 (33): 11927–32. Bibcode:2008PNAS..10511927G. doi:10.1073/pnas.0711752105. PMC 2575260. PMID 18697947.
- Smaldone GT, Antelmann H, Gaballa A, Helmann JD (May 2012). "The FsrA sRNA and FbpB protein mediate the iron-dependent induction of the Bacillus subtilis lutABC iron-sulfur-containing oxidases". Journal of Bacteriology. 194 (10): 2586–93. doi:10.1128/JB.05567-11. PMC 3347220. PMID 22427629.
- Saito Y, Taguchi H, Akamatsu T (March 2006). "Fate of transforming bacterial genome following incorporation into competent cells of Bacillus subtilis: a continuous length of incorporated DNA". Journal of Bioscience and Bioengineering. 101 (3): 257–62. doi:10.1263/jbb.101.257. PMID 16716928.
- Saito Y, Taguchi H, Akamatsu T (April 2006). "DNA taken into Bacillus subtilis competent cells by lysed-protoplast transformation is not ssDNA but dsDNA". Journal of Bioscience and Bioengineering. 101 (4): 334–39. doi:10.1263/jbb.101.334. PMID 16716942.
- Akamatsu T, Taguchi H (April 2001). "Incorporation of the whole chromosomal DNA in protoplast lysates into competent cells of Bacillus subtilis". Bioscience, Biotechnology, and Biochemistry. 65 (4): 823–29. doi:10.1271/bbb.65.823. PMID 11388459. S2CID 30118947.
- Anagnostopoulos C, Spizizen J (May 1961). "Requirements for Transformation in Bacillus Subtilis". Journal of Bacteriology. 81 (5): 741–46. doi:10.1128/JB.81.5.741-746.1961. PMC 279084. PMID 16561900.
- Hoelzer MA, Michod RE (June 1991). "DNA repair and the evolution of transformation in Bacillus subtilis. III. Sex with damaged DNA". Genetics. 128 (2): 215–23. doi:10.1093/genetics/128.2.215. PMC 1204460. PMID 1906416.
- Michod RE, Wojciechowski MF, Hoelzer MA (January 1988). "DNA repair and the evolution of transformation in the bacterium Bacillus subtilis". Genetics. 118 (1): 31–39. doi:10.1093/genetics/118.1.31. PMC 1203263. PMID 8608929.
- Wojciechowski MF, Hoelzer MA, Michod RE (March 1989). "DNA repair and the evolution of transformation in Bacillus subtilis. II. Role of inducible repair". Genetics. 121 (3): 411–22. doi:10.1093/genetics/121.3.411. PMC 1203629. PMID 2497048.
- Michod RE, Bernstein H, Nedelcu AM (May 2008). "Adaptive value of sex in microbial pathogens". Infection, Genetics and Evolution. 8 (3): 267–85. doi:10.1016/j.meegid.2008.01.002. PMID 18295550.
- Itaya M, Nagasaku M, Shimada T, Ohtani N, Shiwa Y, Yoshikawa H, et al. (February 2019). "Stable and efficient delivery of DNA to Bacillus subtilis (natto) using pLS20 conjugational transfer plasmids". FEMS Microbiology Letters. 366 (4). doi:10.1093/femsle/fnz032. PMID 30726909.
- "Chemistry AU naturel: mimicking nature's clean and efficient ways. - Free Online Library". www.thefreelibrary.com. Retrieved 2023-04-29.
- Ciprandi G, Scordamaglia A, Venuti D, Caria M, Canonica GW (December 1986). "In vitro effects of Bacillus subtilis on the immune response". Chemioterapia. 5 (6): 404–07. PMID 3100070.
- Shylakhovenko VA (June 2003). "Anticancer and Immunostimulatory effects of Nucleoprotein Fraction of 'Bacillus subtilis'". Experimental Oncology. 25: 119–23.
- A study of the vulnerability of subway passengers in New York City to covert action with biological agents. Miscellaneous publication. Department of the Army, Fort Detrick. 1968.
- Rosoff S, Pontell H, Tillman R (2020). Profit Without Honor: White Collar Crime and the Looting of America. Pearson. pp. 352–3. ISBN 9780134871486.
- Podstawka A. "Bacillus licheniformis Tracy I | DSM 603, ATCC 10716, CCM 2181, IFO 12199, NBRC 12199, NCIB 8874, FDA BT1 | BacDiveID:686". bacdive.dsmz.de.
- Johnson BA, Anker H, Meleney FL (October 1945). "Bacitracin: a new antibiotic produced by a member of the B. subtilis group". Science. 102 (2650): 376–7. Bibcode:1945Sci...102..376J. doi:10.1126/science.102.2650.376. PMID 17770204. S2CID 51066.
- Horneck G, Klaus DM, Mancinelli RL (March 2010). "Space microbiology". Microbiology and Molecular Biology Reviews. 74 (1): 121–56. Bibcode:2010MMBR...74..121H. doi:10.1128/mmbr.00016-09. PMC 2832349. PMID 20197502.
- Dose K, Bieger-Dose A, Dillmann R, Gill M, Kerz O, Klein A, et al. (1995). "ERA-experiment "Space Biochemistry"". Advances in Space Research. 16 (8): 119–29. Bibcode:1995AdSpR..16h.119D. doi:10.1016/0273-1177(95)00280-R. PMID 11542696.
- Vaisberg O, Fedorov A, Dunjushkin F, Kozhukhovsky A, Smirnov V, Avanov L, et al. (1995). "Ion populations in the tail of Venus". Advances in Space Research. 16 (4): 105–18. Bibcode:1995AdSpR..16d.105V. doi:10.1016/0273-1177(95)00217-3.
- Clancy P (Jun 23, 2005). Looking for Life, Searching the Solar System. Cambridge University Press.
- Horneck G, Klaus DM, Mancinelli RL (March 2010). "Space microbiology". Microbiology and Molecular Biology Reviews. 74 (1): 121–56. Bibcode:2010MMBR...74..121H. doi:10.1128/MMBR.00016-09. PMC 2832349. PMID 20197502.
- Fajardo-Cavazos P, Link L, Melosh HJ, Nicholson WL (December 2005). "Bacillus subtilis spores on artificial meteorites survive hypervelocity atmospheric entry: implications for Lithopanspermia". Astrobiology. 5 (6): 726–36. Bibcode:2005AsBio...5..726F. doi:10.1089/ast.2005.5.726. PMID 16379527.
- Brandstätter F, Brack A, Baglioni P, Cockell CS, Demets R, Edwards HG, et al. (2008). "Mineralogical alteration of artificial meteorites during atmospheric entry. The STONE-5 experiment". Planetary and Space Science. 56 (7): 976–84. Bibcode:2008P&SS...56..976B. CiteSeerX 10.1.1.549.4307. doi:10.1016/j.pss.2007.12.014.
- Wassmann M, Moeller R, Rabbow E, Panitz C, Horneck G, Reitz G, et al. (May 2012). "Survival of spores of the UV-resistant Bacillus subtilis strain MW01 after exposure to low-earth orbit and simulated martian conditions: data from the space experiment ADAPT on EXPOSE-E". Astrobiology. 12 (5): 498–507. Bibcode:2012AsBio..12..498W. doi:10.1089/ast.2011.0772. PMID 22680695.
- Zeigler DR, Prágai Z, Rodriguez S, Chevreux B, Muffler A, Albert T, et al. (November 2008). "The origins of 168, W23, and other Bacillus subtilis legacy strains". Journal of Bacteriology. 190 (21): 6983–95. doi:10.1128/JB.00722-08. PMC 2580678. PMID 18723616.
- Nakamura LK (1989). "Taxonomic Relationship of Black-Pigmented Bacillus subtilis Strains and a Proposal for Bacillus atrophaeus sp. nov". International Journal of Systematic Bacteriology. 39 (3): 295–300. doi:10.1099/00207713-39-3-295.
- Burke SA, Wright JD, Robinson MK, Bronk BV, Warren RL (May 2004). "Detection of molecular diversity in Bacillus atrophaeus by amplified fragment length polymorphism analysis". Applied and Environmental Microbiology. 70 (5): 2786–90. Bibcode:2004ApEnM..70.2786B. doi:10.1128/AEM.70.5.2786-2790.2004. PMC 404429. PMID 15128533.
- "Project 112/SHAD - Shipboard Hazard and Defense". U.S. Department of Veterans' Affairs. Archived from the original on 21 February 2015. Retrieved 25 February 2015.
- Gibbons HS, Broomall SM, McNew LA, Daligault H, Chapman C, Bruce D, Karavis M, Krepps M, McGregor PA, Hong C, Park KH, Akmal A, Feldman A, Lin JS, Chang WE, Higgs BW, Demirev P, Lindquist J, Liem A, Fochler E, Read TD, Tapia R, Johnson S, Bishop-Lilly KA, Detter C, Han C, Sozhamannan S, Rosenzweig CN, Skowronski EW (March 2011). "Genomic signatures of strain selection and enhancement in Bacillus atrophaeus var. globigii, a historical biowarfare simulant". PLOS ONE. 6 (3): e17836. Bibcode:2011PLoSO...617836G. doi:10.1371/journal.pone.0017836. PMC 3064580. PMID 21464989.
- van Dijl JM, Hecker M (January 2013). "Bacillus subtilis: from soil bacterium to super-secreting cell factory". Microbial Cell Factories. 12 (3): 3. doi:10.1186/1475-2859-12-3. PMC 3564730. PMID 23311580.
- "Monilinia fructicola" (PDF). Data Sheets on Quarantine Pests. European Public Prosecutor's Office (EPPO). Archived from the original (PDF) on 2015-06-04. Retrieved 2015-07-21.
- Swain MR, Ray RC (2009). "Biocontrol and other beneficial activities of Bacillus subtilis isolated from cowdung microflora". Microbiological Research. 164 (2): 121–30. doi:10.1016/j.micres.2006.10.009. PMID 17320363.
- Yánez-Mendizábal V (2011). "Biological control of peach brown rot (Monilinia spp.) by Bacillus subtilis CPA-8 is based on production of fengycin-like lipopeptides". European Journal of Plant Pathology. 132 (4): 609–19. doi:10.1007/s10658-011-9905-0. S2CID 15761522.
- Sharaf-Eldin M, Elkholy S, Fernández JA, Junge H, Cheetham R, Guardiola J, Weathers P (August 2008). "Bacillus subtilis FZB24 affects flower quantity and quality of saffron (Crocus sativus)". Planta Medica. 74 (10): 1316–20. doi:10.1055/s-2008-1081293. PMC 3947403. PMID 18622904.
- "The International Pharmacopoeia – Fourth Supplement: Methods of Analysis: 5. Pharmaceutical technical procedures: 5.8 Methods of sterilization". Archived from the original on December 8, 2008.
- "AN-2203 Biological Indicator for EO (25/box)". Andersen Products. Archived from the original on 2013-10-06. Retrieved 2013-05-24.
- Ngugi HK, Dedej S, Delaplane KS, Savelle AT, Scherm H (2005-04-01). "Effect of flower-applied Serenade biofungicide (Bacillus subtilis) on pollination-related variables in rabbiteye blueberry". Biological Control. 33 (1): 32–38. doi:10.1016/j.biocontrol.2005.01.002. ISSN 1049-9644.
- Yu AC, Yim AK, Mat WK, Tong AH, Lok S, Xue H, Tsui SK, Wong JT, Chan TF (March 2014). "Mutations enabling displacement of tryptophan by 4-fluorotryptophan as a canonical amino acid of the genetic code". Genome Biology and Evolution. 6 (3): 629–41. doi:10.1093/gbe/evu044. PMC 3971595. PMID 24572018.
- "Sodium hyaluronate frequently asked questions, hyaluronic acid FAQs, HA – Hyasis® | Novozymes Biopharma". Archived from the original on 2013-08-28. Retrieved 2013-08-13.
- Harrigan GG, Ridley WP, Miller KD, Sorbet R, Riordan SG, Nemeth MA, et al. (October 2009). "The forage and grain of MON 87460, a drought-tolerant corn hybrid, are compositionally equivalent to that of conventional corn". Journal of Agricultural and Food Chemistry. 57 (20): 9754–63. doi:10.1021/jf9021515. PMID 19778059.
- USDA: Determination of Nonregulated Status for MON 87460 Corn (Zea mays L)
- Blum B (2019-11-17). "Israeli students win award for making honey without bees". Israel21c. Retrieved 2019-11-24.
- "Rope Spoilage | Baking Processes". BAKERpedia. Retrieved 2021-02-07.
- Pepe O, Blaiotta G, Moschetti G, Greco T, Villani F (April 2003). "Rope-producing strains of Bacillus spp. from wheat bread and strategy for their control by lactic acid bacteria". Applied and Environmental Microbiology. 69 (4): 2321–9. Bibcode:2003ApEnM..69.2321P. doi:10.1128/AEM.69.4.2321-2329.2003. PMC 154770. PMID 12676716.
- Lefevre M, Racedo SM, Denayrolles M, Ripert G, Desfougères T, Lobach AR, et al. (February 2017). "Safety assessment of Bacillus subtilis CU1 for use as a probiotic in humans". Regulatory Toxicology and Pharmacology. 83: 54–65. doi:10.1016/j.yrtph.2016.11.010. PMID 27825987.
- "FDA partial list of microorganisms". Food and Drug Administration. 2002.
- Shortt C (September 2005). "Perspectives on foods for specific health uses (FOSHU)". In Gibson GR (ed.). Food Science and Technology Bulletin : Functional Foods. Vol. 1. Reading: IFIS Publishing. pp. 7–1. ISBN 978-0-86014-193-8.
- EFSA Panel on Biological Hazards (BIOHAZ) (2010). "Scientific opinion on the maintenance of the list of QPS microorganisms intentionally added to food or feed (2010 update)". EFSA Journal. 8 (12): 1944. doi:10.2903/j.efsa.2010.1944.
External links
 Media related to Bacillus subtilis at Wikimedia Commons
Media related to Bacillus subtilis at Wikimedia Commons- SubtiWiki "up-to-date information for all genes of Bacillus subtilis"
- Bacillus subtilis Final Risk Assessment on EPA.gov. Archived from the original on 2015-09-09.
- Bacillus subtilis genome browser
- Type strain of Bacillus subtilis at BacDive - the Bacterial Diversity Metadatabase