Barton–Zard reaction
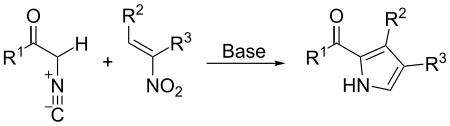
The Barton–Zard reaction is a route to pyrrole derivatives via the reaction of a nitroalkene with an α-isocyanoacetate under basic conditions.[1] It is named after Derek Barton and Samir Zard who first reported it in 1985.[2]
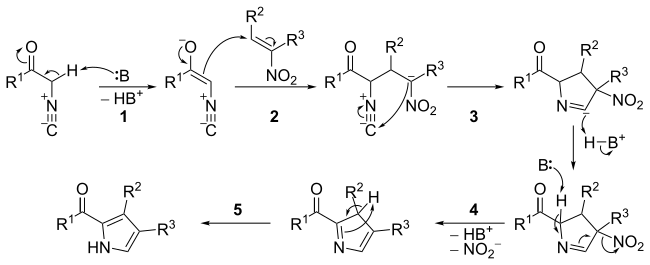
Mechanism
The mechanism consists of five steps:
- Base catalyzed carbonyl enolization of the α-isocyanide.
- Michael-type addition between the α-isocyanide carbonyl enolate and the nitroalkene.
- 5-endo-dig cyclization (see: Baldwin's rules).
- Base catalyzed elimination of the nitro group.
- Tautomerization leading to aromatisation.
Scope
The nitro compound may be aromatic rather than just an alkene.[3] The reaction has been used for the synthesis of polypyrroles, including porphyrins,[4] as well as dipyrromethenes such as BODIPY.[5]
References
- Jie Jack Li (2013). Heterocyclic Chemistry in Drug Discovery. New York: Wiley. ISBN 9781118354421. pp.43-4
- Barton, Derek H. R.; Zard, Samir Z. (1985). "A new synthesis of pyrroles from nitroalkenes". Journal of the Chemical Society, Chemical Communications (16): 1098. doi:10.1039/C39850001098.
- Lash, Timothy D.; Novak, Bennett H.; Lin, Yanning (April 1994). "Synthesis of phenanthropyrroles and phenanthrolinopyrroles from isocyanoacetates: An extension of the barton-zard pyrrole condensation". Tetrahedron Letters. 35 (16): 2493–2494. doi:10.1016/S0040-4039(00)77152-8.
- Finikova, Olga S.; Cheprakov, Andrei V.; Beletskaya, Irina P.; Carroll, Patrick J.; Vinogradov, Sergei A. (January 2004). "Novel Versatile Synthesis of Substituted Tetrabenzoporphyrins". The Journal of Organic Chemistry. 69 (2): 522–535. doi:10.1021/jo0350054. PMID 14725469.
- Ono, Noboru (2008). "Barton-Zard Pyrrole Synthesis and Its Application to Synthesis of Porphyrins, Polypyrroles, and Dipyrromethene Dyes". Heterocycles. 75 (2): 243. doi:10.3987/REV-07-622.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.