Bredt's rule
In organic chemistry, Bredt's rule is an empirical observation that states that a double bond cannot be placed at the bridgehead of a bridged ring system, unless the rings are large enough. The rule is named after Julius Bredt, who first discussed it in 1902[1] and codified it in 1924.[2] It primarily relates to bridgeheads with carbon-carbon and carbon-nitrogen double bonds.[3]
For example, two of the following isomers of norbornene violate Bredt's rule, which makes them too unstable to prepare:
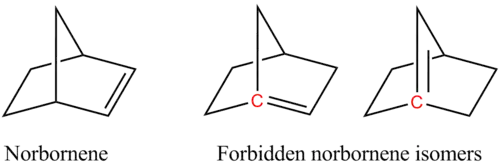
In the figure, the bridgehead atoms involved in Bredt's rule violation are highlighted in red.
Bredt's rule is a consequence of the fact that having a double bond on a bridgehead, carbons from which three bonds radiate and which the rings share a single covalent bond, would be equivalent to having a trans double bond on a ring, which is not stable for small rings (fewer than eight atoms) due to a combination of ring strain, and angle strain (nonplanar alkene). The p orbitals of the bridgehead atom and adjacent atoms are orthogonal and thus are not aligned properly for the formation of pi bonds. Fawcett quantified the rule by defining S as the number of non-bridgehead atoms in a ring system, and postulated that stability required S ≥ 9 in bicyclic systems[4] and S ≥ 11 in tricyclic systems.[5] There has been an active research program to seek compounds inconsistent with the rule,[6] and for bicyclic systems a limit of S ≥ 7 is now established[3] with several such compounds having been prepared.[7] The above norbornene system has S = 5 and so they are not preparable.
Bredt's rule can be useful for predicting which isomer is obtained from an elimination reaction in a bridged ring system. It can also be applied to reaction mechanisms that go via carbocations and, to a lesser degree, via free radicals, because these intermediates, like carbon atoms involved in a double bond, prefer to have a planar geometry with 120 degree angles and sp2 hybridization. The rule also allows the rationalisation of observations. For example, bicyclo[5.3.1]undecane-11-one-1-carboxylic acid undergoes decarboxylation on heating to 132 °C, but the similar compound bicyclo[2.2.1]heptan-7-one-1-carboxylic acid remains stable beyond 500 °C, despite both being β-keto acids with the carbonyl group on a one-carbon bridge and the carboxylate group on the bridgehead. The mechanism of decarboxylation involves an enolate intermediate, which is an S = 9 species in the former case and an S = 5 species in the latter, preventing the decarboxylation in the smaller ring system.[3]
An anti-bredt molecule is one that is found to exist and be stable (within certain parameters) despite this rule. A recent (2006) example of such a molecule is 2-quinuclidonium tetrafluoroborate.[8] Bridgehead double bonds can be found in some natural products, discussed in a review by Mak, Pouwer and Williams,[9] and an older review by Shea looked at bridgehead alkenes more generally.[10]
See also
- Metal tetranorbornyls, which are stabilized by Bredt's rule
References
- Bredt, J.; Houben, Jos.; Levy, Paul (1902). "Ueber isomere Dehydrocamphersäuren, Lauronolsäuren und Bihydrolauro-Lactone". Ber. Dtsch. Chem. Ges. (in German). 35 (2): 1286–1292. doi:10.1002/cber.19020350215.
- Bredt, J. (1924). "Über sterische Hinderung in Brückenringen (Bredtsche Regel) und über die meso-trans-Stellung in kondensierten Ringsystemen des Hexamethylens". Justus Liebigs Ann. Chem. (in German). 437 (1): 1–13. doi:10.1002/jlac.19244370102.
- Bansal, Raj K. (1998). "Bredt's Rule". Organic Reaction Mechanisms (3rd ed.). McGraw-Hill Education. pp. 14–16. ISBN 9780074620830.
- Fawcett, Frank S. (1950). "Bredt's Rule of Double Bonds in Atomic-Bridged-Ring Structures". Chem. Rev. 47 (2): 219–274. doi:10.1021/cr60147a003. PMID 24538877.
- "Bredt's Rule". Comprehensive Organic Name Reactions and Reagents. 116: 525–528. 2010. doi:10.1002/9780470638859.conrr116. ISBN 9780470638859.
- Köbrich, Gert (1973). "Bredt Compounds and the Bredt Rule". Angew. Chem. Int. Ed. 12 (6): 464–473. doi:10.1002/anie.197304641.
- Hall, H. K.; El-Shekeil, Ali (1980). "Anti-Bredt molecules. 3. 3-Oxa-1-azabicyclo[3.3.1]nonan-2-one and 6-oxa-1-azabicyclo[3.2.1]octan-7-one, two atom-bridged bicyclic urethanes possessing bridgehead nitrogen". J. Org. Chem. 45 (26): 5325–5328. doi:10.1021/jo01314a022.
- Tani, Kousuke; Stoltz, Brian M. (2006). "Synthesis and structural analysis of 2-quinuclidonium tetrafluoroborate" (PDF). Nature. 441 (7094): 731–734. Bibcode:2006Natur.441..731T. doi:10.1038/nature04842. PMID 16760973. S2CID 4332059.
- Mak, Jeffrey Y. W.; Pouwer, Rebecca H.; Williams, Craig M. (2014). "Natural Products with Anti-Bredt and Bridgehead Double Bonds" (PDF). Angew. Chem. Int. Ed. 53 (50): 13664–13688. doi:10.1002/anie.201400932. PMID 25399486.
- Shea, Kenneth J. (1980). "Recent developments in the synthesis, structure and chemistry of bridgehead alkenes". Tetrahedron. 36 (12): 1683–1715. doi:10.1016/0040-4020(80)80067-6.