Caesium superoxide
Caesium superoxide is the superoxide of caesium. It is an orange solid.
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| |
| |
| Properties | |
| CsO2 | |
| Molar mass | 164.903 g·mol−1 |
| Appearance | yellow to orange solid [1] |
| Density | 3.77 g·cm−3[1] |
| Melting point | 600 °C[2] |
| reacts | |
| Related compounds | |
Other anions |
caesium oxide caesium peroxide |
Other cations |
lithium superoxide sodium superoxide potassium superoxide rubidium superoxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Properties
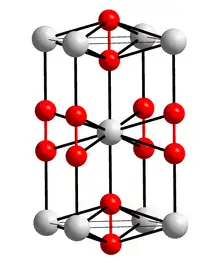
Caesium superoxide's crystal structure is same as calcium carbide. It contains direct oxygen-oxygen bonding.[2]
It reacts with water to form hydrogen peroxide and caesium hydroxide.[2]
- 2 CsO2 + 2H2O → O2↑ + H2O2 + 2 CsOH
Heating to approximately 400 °C induces thermal decomposition to caesium peroxide.[3]
The standard enthalpy of formation ΔHf0 of caesium superoxide is −295 kJ/mol.[4]
Caesium superoxide reacts with ozone to form caesium ozonide.[2]
- CsO2 + O3 → CsO3 + O2
References
- Caesiumhyperoxid bei webelements.com.
- Holleman, Arnold (2007). Lehrbuch der anorganischen Chemie (in German). BerlinNew York: de Gruyter. ISBN 978-3-11-017770-1. OCLC 180963521.
- Berardinelli, S. P.; Kraus, D. L. (1974-01-01). "Thermal decomposition of the higher oxides of cesium in the temperature range 320-500.deg". Inorganic Chemistry. American Chemical Society (ACS). 13 (1): 189–191. doi:10.1021/ic50131a037. ISSN 0020-1669.
- Holleman, Arnold (2017). Anorganische ChemienBand 1 (in German). Berlin: de Gruyter. ISBN 978-3-11-049585-0. OCLC 968134975.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.