EZH2
Enhancer of zeste homolog 2 (EZH2) is a histone-lysine N-methyltransferase enzyme (EC 2.1.1.43) encoded by EZH2 gene, that participates in histone methylation and, ultimately, transcriptional repression.[5] EZH2 catalyzes the addition of methyl groups to histone H3 at lysine 27,[6] by using the cofactor S-adenosyl-L-methionine. Methylation activity of EZH2 facilitates heterochromatin formation thereby silences gene function.[5] Remodeling of chromosomal heterochromatin by EZH2 is also required during cell mitosis.
EZH2 is the functional enzymatic component of the Polycomb Repressive Complex 2 (PRC2), which is responsible for healthy embryonic development through the epigenetic maintenance of genes responsible for regulating development and differentiation.[7] EZH2 is responsible for the methylation activity of PRC2, and the complex also contains proteins required for optimal function (EED, SUZ12, JARID2, AEBP2, RbAp46/48, and PCL).[8]
Mutation or over-expression of EZH2 has been linked to many forms of cancer.[9] EZH2 inhibits genes responsible for suppressing tumor development, and blocking EZH2 activity may slow tumor growth. EZH2 has been targeted for inhibition because it is upregulated in multiple cancers including, but not limited to, breast,[10] prostate,[11] melanoma,[12] and bladder cancer.[13] Mutations in the EZH2 gene are also associated with Weaver syndrome, a rare congenital disorder,[14] and EZH2 is involved in causing neurodegenerative symptoms in the nervous system disorder, ataxia telangiectasia.[15]
Function
| Histone-lysine N-methyltransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 2.1.1.43 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
EZH2 is the catalytic subunit of the Polycomb Repressive Complex 2 (PRC2).[16] EZH2's catalytic activity relies on its formation of a complex with at least two other PRC2 components, SUZ12 and EED.[17]
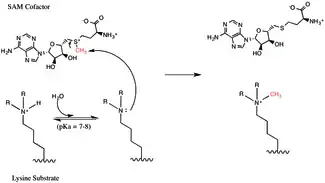
As a histone methyltransferase (HMTase), EZH2's primary function is to methylate Lys-27 on histone 3 (H3K27me) by transferring a methyl group from the cofactor S-adenosyl-L-methionine (SAM). EZH2 is capable of mono-, di-, and tri-methylation of H3K27 and has been associated with a variety of biological functions, including transcriptional regulation in hematopoiesis, development, and cell differentiation.[17][18][19][20]
Recent studies have indicated that EZH2 is also capable of methylating non-histone proteins.[17][18]
Transcription repression
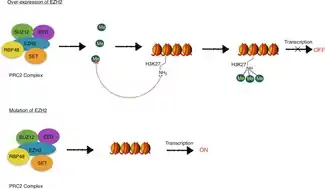
EZH2, as a part of PRC2, catalyzes trimethylation of H3K27 (H3K27me3), which is a histone modification that has been characterized as part of the histone code.[16][20][21][22] The histone code is the theory that chemical modifications, such as methylation, acetylation, and ubiquitination, of histone proteins play distinctive roles in epigenetic regulation of gene transcription. EZH2-mediated catalysis of H3K27me3 is associated with long term transcription repression.[16][20][21]
EZH2, as well as other Polycomb group proteins, are involved in establishing and maintaining gene repression through cell division.[17][20] This transcriptionally repressive state is thought to be due to PRC2/EZH2-EED-mediated H3K27 methylation and subsequent recruitment of PRC1 which facilitates condensation of chromatin and formation of heterochromatin.[20] Heterochromatin is tightly packed chromatin which limits the accessibility of transcription machinery to the underlying DNA, thereby suppressing transcription.[23]
During cell division, heterochromatin formation is required for proper chromosome segregation.[24] PRC2/EED-EZH2 complex may also be involved in the recruitment of DNA methyltransferases (DNMTs), which results in increased DNA methylation, another epigenetic layer of transcription repression.[16][17] Specific genes that have been identified as targets of EZH2-mediated transcriptional repression include HOXA9, HOXC8, MYT1, CDKN2A and retinoic acid target genes.[16]
Transcription activation
In cancer, EZH2 may play a role in activation of transcription, independently of PRC2.[17] In breast cancer cells, EZH2 has been demonstrated to activate NF-κB target genes, which are involved in responses to stimuli.[17] The functional role of this activity and its mechanism are still unknown.
Development and cell differentiation
EZH2 plays an essential role in development. In particular, it helps control transcriptional repression of genes that regulate cell differentiation.[17][18][20][21] In embryonic stem cells, EZH2-mediated trimethylation of H3K27me3 in regions containing developmental genes appears to be important for maintenance of normal cell differentiation.[20] H3K27me3 is also important in driving X-inactivation, the silencing of one X-chromosome in females during development.[22] During X-inactivation, it is thought that EZH2 is involved in initiating heterochromatin formation by trimethylating H3K27 and that other histone methyltransferases and histone marks may be involved in maintaining the silenced state.[25]
Further, EZH2 has been identified as an essential protein involved in development and differentiation of B-cells and T-cells.[18] H3K27me3 is involved in suppressing genes that promote differentiation, thus maintaining an undifferentiated state of B- and T-cells and playing an important role in regulating hematopoiesis.[18][26][27]
Regulation of EZH2 activity
The activity of EZH2 is regulated by the post-translational phosphorylation of threonine and serine residues on EZH2.[28] Specifically, phosphorylation of T350 has been linked to an increase in EZH2 activity while phosphorylation of T492 and S21 have been linked to a decrease in EZH2 activity.[21][28] Phosphorylation of T492 has been suggested to disrupt contacts between human EZH2 and its binding partners in the PRC2 complex, thus hindering its catalytic activity.[21]
In addition to phosphorylation, it has also been shown that PRC2/EZH2-EED activity is antagonized by transcription-activating histone marks, such as acetylation of H3K27 (H3K27ac) and methylation of H3K36 (H3K36me).[21][29]
EZH2 expression is regulated by estrogen signaling in human normal breast epithelium and human breast cancers.[30]
Enzymatic activity
EZH2 function is highly dependent upon its recruitment by the PRC2 complex. In particular, WD40-repeat protein embryonic ectoderm development (EED) and zinc finger protein suppressor of zeste 12 (SUZ12) are needed to stabilize the interaction of EZH2 with its histone substrate[31][32] Recently, two isoforms of EZH2 generated from alternative splicing have been identified in humans: EZH2α and EZH2β.[33] Both isoforms contain elements that have been identified as important for EZH2 function including the nuclear localization signal, the EED and SUZ12 binding sites as well as the conserved SET domain.[33] Most studies have thus far focused on the longer isoform EZH2α, but EZH2β, which lacks exons 4 and 8, has been shown to be active.[33] Furthermore, PRC2/EZH2β complexes act on distinct genes from that of its PRC2/EZH2α counterpart suggesting that each isoform may act to regulate a specific subset of genes.[33] Additional evidence suggests that EZH2 may also be capable of lysine methylation independent of association with PRC2, when EZH2 is highly upregulated.[17]
Lysine methylation
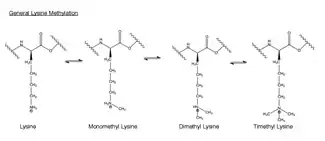
Methylation is the addition of a -CH3, or methyl group, to another molecule. In biology, methylation is typically catalyzed by enzymes, and methyl groups are commonly added to either proteins or nucleic acids. In EZH2-catalyzed methylation, the amino acid lysine in the histone h3 is methylated. This amino acid residue can be methylated up to three times on its terminal ammonium group. These methylated lysines are important in the control of mammalian gene expression and have a functional role in heterochromatin formation, X-chromosome inactivation and transcriptional regulation.[34] In mammalian chromosomes, histone lysine methylation can either activate or repress genes depending the site of methylation. Recent work has shown that at least part of the silencing function of the EZH2 complex is the methylation of histone H3 on lysine 27.[35] Methylation, and other modifications, take place on the histones. Methyl modifications can affect the binding of proteins to these histones and either activate or inhibit transcription.[24]
Mechanism of catalysis
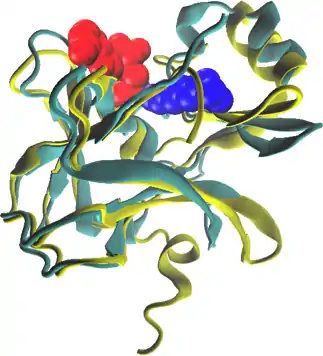
EZH2 is a member of the SET domain family of lysine methyltransferases which function to add methyl groups to lysine side chains of substrate proteins.[36] SET methyltransferases depend on a S-Adenosyl methionine (SAM) cofactor to act as a methyl donor for their catalytic activity. SET domain proteins differ from other SAM-dependent methyltransferases in that they bind their substrate and SAM cofactor on opposite sides of the active site of the enzyme. This orientation of substrate and cofactor allows SAM to dissociate without disrupting substrate binding and can lead to multiple rounds of lysine methylation without substrate dissociation.[36]
Although neither a substrate-bound or SAM-bound crystal structure for EZH2 has been determined, STAMP structure alignment with the human SET7/9 methyltransferase shows conserved tyrosine residues in almost identical positions within the putative active site of EZH2.
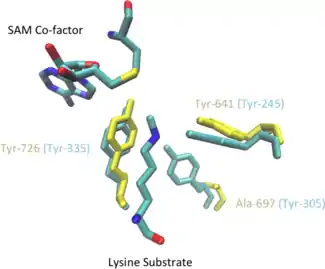
It had been previously suggested that tyrosine 726 in the EZH2 active site was acting as a general base to de-protonate the substrate lysine but kinetic isotope effects have indicated that active site residues are not directly involved in the chemistry of the methyltransferase reaction.[37] Instead these experiments support a mechanism in which the residues lower the pKa of the substrate lysine residue while simultaneously providing a channel for water to access the lysine side chain within the interior of the active site. Bulk solvent water can then easily deprotonate the lysine side chain, activating it for nucleophilic attack of the SAM cofactor in an SN2-like reaction resulting in transfer of the methyl group from SAM to the lysine side chain.[37]
EZH2 primarily catalyzes mono- and di-methylation of H3K27 but a clinically relevant mutation of residue tyrosine 641 to phenylalanine (Y641F) results in higher H3K27 tri-methylation activity.[37][38] It is proposed that the removal of the hydroxyl group on Y641 abrogates steric hindrance and allows for accommodation of a third methyl group on the substrate lysine.
Clinical significance
Cancer
EZH2 is an attractive target for anti-cancer therapy because it helps cancerous cells divide and proliferate. It is found in larger amounts than in healthy cells in a wide range of cancers including breast, prostate, bladder, uterine, and renal cancers, as well as melanoma and lymphoma. EZH2 is a gene suppressor, so when it becomes overexpressed, many tumor suppressor genes that are normally turned on, are turned off. Inhibition of EZH2 function shrinks malignant tumors in some reported cases because those tumor suppressor genes are not silenced by EZH2.[39] EZH2 typically is not expressed in healthy adults; it is only found in actively dividing cells, like those active during fetal development.[40] Because of this characteristic, overexpression of EZH2 can be used as a diagnostic marker of cancer and some neurodegenerative disorders.[15] However, there are cases where it is difficult to tell whether overexpression of EZH2 is the cause of a disease, or simply a consequence. If it is only a consequence, targeting EZH2 for inhibition may not cure the disease. One example of a cancer pathway in which EZH2 plays a role is the pRB-E2F pathway. It is downstream from the pRB-E2F pathway, and signals from this pathway lead to EZH2 overexpression.[41] Another important characteristic of EZH2 is that when EZH2 is overexpressed, it can activate genes without forming PRC2. This is an issue because it means the methylation activity of the enzyme is not mediated by complex formation. In breast cancer cells, EZH2 activates genes that promote cell proliferation and survival.[17] It can also activate regulatory genes like c-myc and cyclin D1 by interacting with Wnt signaling factors.[42] Importantly, the mutation of tyrosine 641 in the active SET domain to a number of different amino acids is a common feature of some B-cell lymphomas.[43]
Inhibitors
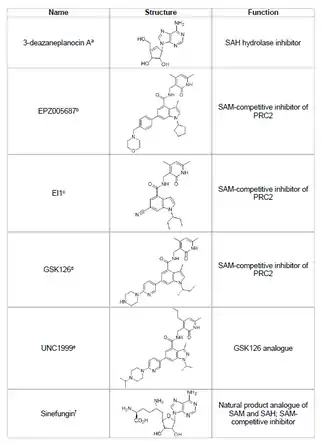
Developing an inhibitor of EZH2 and preventing unwanted histone methylation of tumor suppressor genes is a viable area of cancer research. EZH2 inhibitor development has focused on targeting the SET domain active site of the protein. Several inhibitors of EZH2 have been developed as of 2015, including 3-deazaneplanocin A (DZNep), EPZ005687, EI1, GSK126, and UNC1999.
- DZNep
- DZNep has potential antiviral and anti-cancer properties because it lowers EZH2 levels and induces apoptosis in breast and colon cancer cells.[44] DZNep inhibits the hydrolysis of S-adenosyl-L-homocysteine (SAH), which is a product-based inhibitor of all protein methyltransferases, leading to increased cellular concentrations of SAH which in turn inhibits EZH2. However, DZNep is not specific to EZH2 and also inhibits other DNA methyltransferases.
- EPZ005687
- In 2012, a company called Epizyme revealed EPZ005687, an S-adenosylmethionine (SAM) competitive inhibitor that is more selective than DZNep; it has a 50-fold increase in selectivity for EZH2 compared to EZH1. The drug blocks EZH2 activity by binding to the SET domain active site of the enzyme. EPZ005687 can also inhibit the Y641 and A677 mutants of EZH2, which may be applicable for treating non-Hodgkin's lymphoma.[45]
- Tazemetostat
- In 2013, Epizyme began Phase I clinical trials with another EZH2 inhibitor, tazemetostat (EPZ-6438), for patients with B-cell lymphoma.[49] In 2020, tazemetostat, with the tradename Tazverik, gained an FDA accelerated approval for the treatment of metastatic or locally advanced epithelioid sarcoma and an accelerated approval for the treatment of patients with relapsed follicular lymphoma later that year.[50]
- Sinefungin
- Sinefungin is another SAM-competitive inhibitor, however, like DZNep, it is not specific to EZH2.[48] It works by binding in the cofactor binding pocket of DNA methyltransferases to block methyl transfer. EI1 is another inhibitor, developed by Novartis, that showed EZH2 inhibitory activity in lymphoma tumor cells, including cells with the Y641 mutation.[46] The mechanism of this inhibitor also involves competing with the SAM cofactor for binding to EZH2.[46]
- GSK126
- GSK126 is a potent, SAM-competitive EZH2 inhibitor developed by GlaxoSmithKline, that has 150-fold selectivity over EZH1 and a Ki of 0.5-3 nM.[47] UNC1999 was developed as an analogue of GSK126, and was the first orally bioavailable EZH2 inhibitor to show activity. However, it is less selective than its counterpart GSK126, and it binds to EZH1 as well, increasing the potential for off-target effects.
Combination therapies are being studied as possible treatments when primary treatments begin to fail. Etoposide, a topoisomerase inhibitor, when combined with an EZH2 inhibitor, becomes more effective for non-small cell lung cancers with BRG1 and EGFR mutations.[39] However, EZH2 and lysine methylation can have tumor suppressing activity, for example in myelodysplastic syndrome,[51] indicating that EZH2 inhibition may not be beneficial in all cases.
Weaver Syndrome
Mutations in the EZH2 gene have been linked with Weaver syndrome, a rare disorder characterized by advanced bone age, macrocephaly, and hypertelorism.[14] The histidine residue in the active site of the wild-type EZH2 was mutated to tyrosine in patients diagnosed with Weaver syndrome.[14] The mutation likely interferes with cofactor binding and causes disruption of the natural function of the protein.[14]
Taxonomic distribution
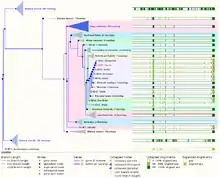
Enhancer of zeste (E(z)) was originally identified in Drosophila melanogaster, and its mammalian homologs were subsequently identified and named EZH1 (enhancer of zeste homolog 1) and EZH2 (enhancer of zeste homolog 2).[53] EZH2 is highly conserved through evolution. It and its homologs play essential roles in development, cell differentiation, and cell division in plants, insects, fish, and mammals.[17][21][54][55] The following taxonomic tree is a depiction of EZH2's distribution throughout a wide variety of species.[56][57]
See also
References
- GRCh38: Ensembl release 89: ENSG00000106462 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000029687 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Viré E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, et al. (February 2006). "The Polycomb group protein EZH2 directly controls DNA methylation". Nature. 439 (7078): 871–874. Bibcode:2006Natur.439..871V. doi:10.1038/nature04431. PMID 16357870. S2CID 4409726.
- Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. (November 2002). "Role of histone H3 lysine 27 methylation in Polycomb-group silencing". Science. 298 (5595): 1039–1043. Bibcode:2002Sci...298.1039C. doi:10.1126/science.1076997. PMID 12351676. S2CID 6265267.
- Morey L, Helin K (June 2010). "Polycomb group protein-mediated repression of transcription". Trends in Biochemical Sciences. 35 (6): 323–332. doi:10.1016/j.tibs.2010.02.009. PMID 20346678.
- Margueron R, Reinberg D (January 2011). "The Polycomb complex PRC2 and its mark in life". Nature. 469 (7330): 343–349. Bibcode:2011Natur.469..343M. doi:10.1038/nature09784. PMC 3760771. PMID 21248841.
- Kim KH, Roberts CW (February 2016). "Targeting EZH2 in cancer". Nature Medicine. 22 (2): 128–134. doi:10.1038/nm.4036. PMC 4918227. PMID 26845405.
- Yoo KH, Hennighausen L (2012). "EZH2 methyltransferase and H3K27 methylation in breast cancer". International Journal of Biological Sciences. 8 (1): 59–65. doi:10.7150/ijbs.8.59. PMC 3226033. PMID 22211105.
- Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, et al. (October 2002). "The polycomb group protein EZH2 is involved in progression of prostate cancer". Nature. 419 (6907): 624–629. Bibcode:2002Natur.419..624V. doi:10.1038/nature01075. hdl:2027.42/62896. PMID 12374981. S2CID 4414767.
- Sarah Graham (October 10, 2002). "Scientists Identify Gene That Marks Deadliest Form of Prostate Cancer". Scientific American.
- Zingg D, Debbache J, Schaefer SM, Tuncer E, Frommel SC, Cheng P, et al. (January 2015). "The epigenetic modifier EZH2 controls melanoma growth and metastasis through silencing of distinct tumour suppressors". Nature Communications. 6: 6051. Bibcode:2015NatCo...6.6051Z. doi:10.1038/ncomms7051. PMID 25609585.
- "25 Jan Epigenetic Control Protein Allows Melanoma Cells to Metatasize". MedicalResearch.
- Arisan S, Buyuktuncer ED, Palavan-Unsal N, Caşkurlu T, Cakir OO, Ergenekon E (2005). "Increased expression of EZH2, a polycomb group protein, in bladder carcinoma". Urologia Internationalis. 75 (3): 252–257. doi:10.1159/000087804. PMID 16215315. S2CID 26843362.
- Gibson WT, Hood RL, Zhan SH, Bulman DE, Fejes AP, Moore R, et al. (January 2012). "Mutations in EZH2 cause Weaver syndrome". American Journal of Human Genetics. 90 (1): 110–118. doi:10.1016/j.ajhg.2011.11.018. PMC 3257956. PMID 22177091.
- Li J, Hart RP, Mallimo EM, Swerdel MR, Kusnecov AW, Herrup K (December 2013). "EZH2-mediated H3K27 trimethylation mediates neurodegeneration in ataxia-telangiectasia". Nature Neuroscience. 16 (12): 1745–1753. doi:10.1038/nn.3564. PMC 3965909. PMID 24162653.
- Universal protein resource accession number Q15910 at UniProt.
- Tan JZ, Yan Y, Wang XX, Jiang Y, Xu HE (February 2014). "EZH2: biology, disease, and structure-based drug discovery". Acta Pharmacologica Sinica. 35 (2): 161–174. doi:10.1038/aps.2013.161. PMC 3914023. PMID 24362326.
- Lund K, Adams PD, Copland M (January 2014). "EZH2 in normal and malignant hematopoiesis". Leukemia. 28 (1): 44–49. doi:10.1038/leu.2013.288. PMID 24097338. S2CID 736796.
- "RefSeq". RefSeq Gene EZH2. Retrieved February 1, 2015.
- Ding X, Wang X, Sontag S, Qin J, Wanek P, Lin Q, Zenke M (May 2014). "The polycomb protein Ezh2 impacts on induced pluripotent stem cell generation". Stem Cells and Development. 23 (9): 931–940. doi:10.1089/scd.2013.0267. PMC 3996971. PMID 24325319.
- O'Meara MM, Simon JA (June 2012). "Inner workings and regulatory inputs that control Polycomb repressive complex 2". Chromosoma. 121 (3): 221–234. doi:10.1007/s00412-012-0361-1. PMC 3351537. PMID 22349693.
- "Histone H3K27". EpiGenie.
- Grewal SI, Jia S (January 2007). "Heterochromatin revisited". Nature Reviews. Genetics. 8 (1): 35–46. doi:10.1038/nrg2008. PMID 17173056. S2CID 31811880.
- Stewart MD, Li J, Wong J (April 2005). "Relationship between histone H3 lysine 9 methylation, transcription repression, and heterochromatin protein 1 recruitment". Molecular and Cellular Biology. 25 (7): 2525–2538. doi:10.1128/MCB.25.7.2525-2538.2005. PMC 1061631. PMID 15767660.
- Jeanteur P (2008). Epigenetics and Chromatin. Springer. ISBN 9783540852360.
- Neo WH, Booth CA, Azzoni E, Chi L, Delgado-Olguín P, de Bruijn MF, et al. (May 2018). "Cell-extrinsic hematopoietic impact of Ezh2 inactivation in fetal liver endothelial cells". Blood. 131 (20): 2223–2234. doi:10.1182/blood-2017-10-811455. PMC 5960588. PMID 29555646.
- Neo WH, Meng Y, Rodriguez-Meira A, Fadlullah MZ, Booth CA, Azzoni E, et al. (December 2021). "Ezh2 is essential for the generation of functional yolk sac derived erythro-myeloid progenitors". Nature Communications. 12 (1): 7019. doi:10.1038/s41467-021-27140-8. hdl:10281/339795. PMID 34857757.
- Kaneko S, Li G, Son J, Xu CF, Margueron R, Neubert TA, Reinberg D (December 2010). "Phosphorylation of the PRC2 component Ezh2 is cell cycle-regulated and up-regulates its binding to ncRNA". Genes & Development. 24 (23): 2615–2620. doi:10.1101/gad.1983810. PMC 2994035. PMID 21123648.
- Tie F, Banerjee R, Stratton CA, Prasad-Sinha J, Stepanik V, Zlobin A, et al. (September 2009). "CBP-mediated acetylation of histone H3 lysine 27 antagonizes Drosophila Polycomb silencing". Development. 136 (18): 3131–3141. doi:10.1242/dev.037127. PMC 2730368. PMID 19700617.
- Osako T, Lee H, Turashvili G, Chiu D, McKinney S, Joosten SE, et al. (May 2020). "Age-correlated protein and transcript expression in breast cancer and normal breast tissues is dominated by host endocrine effects". Nature Cancer. 1 (5): 518–532. doi:10.1038/s43018-020-0060-4. PMID 35121983. S2CID 218955089.
- Cao R, Zhang Y (July 2004). "SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED-EZH2 complex". Molecular Cell. 15 (1): 57–67. doi:10.1016/j.molcel.2004.06.020. PMID 15225548.
- Denisenko O, Shnyreva M, Suzuki H, Bomsztyk K (October 1998). "Point mutations in the WD40 domain of Eed block its interaction with Ezh2". Molecular and Cellular Biology. 18 (10): 5634–5642. doi:10.1128/MCB.18.10.5634. PMC 109149. PMID 9742080.
- Grzenda A, Lomberk G, Svingen P, Mathison A, Calvo E, Iovanna J, et al. (February 2013). "Functional characterization of EZH2β reveals the increased complexity of EZH2 isoforms involved in the regulation of mammalian gene expression". Epigenetics & Chromatin. 6 (1): 3. doi:10.1186/1756-8935-6-3. PMC 3606351. PMID 23448518.
- Martin C, Zhang Y (November 2005). "The diverse functions of histone lysine methylation". Nature Reviews. Molecular Cell Biology. 6 (11): 838–849. doi:10.1038/nrm1761. PMID 16261189. S2CID 31300025.
- Brien GL, Gambero G, O'Connell DJ, Jerman E, Turner SA, Egan CM, et al. (December 2012). "Polycomb PHF19 binds H3K36me3 and recruits PRC2 and demethylase NO66 to embryonic stem cell genes during differentiation". Nature Structural & Molecular Biology. 19 (12): 1273–1281. doi:10.1038/nsmb.2449. hdl:2262/97536. PMID 23160351. S2CID 1017805.
- Dillon SC, Zhang X, Trievel RC, Cheng X (2005). "The SET-domain protein superfamily: protein lysine methyltransferases". Genome Biology. 6 (8): 227. doi:10.1186/gb-2005-6-8-227. PMC 1273623. PMID 16086857.
- Kipp DR, Quinn CM, Fortin PD (October 2013). "Enzyme-dependent lysine deprotonation in EZH2 catalysis". Biochemistry. 52 (39): 6866–6878. doi:10.1021/bi400805w. PMID 24000826.
- Yap DB, Chu J, Berg T, Schapira M, Cheng SW, Moradian A, et al. (February 2011). "Somatic mutations at EZH2 Y641 act dominantly through a mechanism of selectively altered PRC2 catalytic activity, to increase H3K27 trimethylation". Blood. 117 (8): 2451–2459. doi:10.1182/blood-2010-11-321208. PMC 3062411. PMID 21190999.
- "Window of Vulnerability". Harvard Medical School.
- Konze KD, Ma A, Li F, Barsyte-Lovejoy D, Parton T, Macnevin CJ, et al. (2013). "An orally bioavailable chemical probe of the Lysine Methyltransferases EZH2 and EZH1". ACS Chemical Biology. 8 (6): 1324–1334. doi:10.1021/cb400133j. PMC 3773059. PMID 23614352.
- Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K (October 2003). "EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer". The EMBO Journal. 22 (20): 5323–5335. doi:10.1093/emboj/cdg542. PMC 213796. PMID 14532106.
- Shi B, Liang J, Yang X, Wang Y, Zhao Y, Wu H, et al. (July 2007). "Integration of estrogen and Wnt signaling circuits by the polycomb group protein EZH2 in breast cancer cells". Molecular and Cellular Biology. 27 (14): 5105–5119. doi:10.1128/MCB.00162-07. PMC 1951944. PMID 17502350.
- Morin RD, Johnson NA, Severson TM, Mungall AJ, An J, Goya R, et al. (February 2010). "Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin". Nature Genetics. 42 (2): 181–185. doi:10.1038/ng.518. PMC 2850970. PMID 20081860.
- Tan J, Yang X, Zhuang L, Jiang X, Chen W, Lee PL, et al. (May 2007). "Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells". Genes & Development. 21 (9): 1050–1063. doi:10.1101/gad.1524107. PMC 1855231. PMID 17437993.
- Knutson SK, Wigle TJ, Warholic NM, Sneeringer CJ, Allain CJ, Klaus CR, et al. (November 2012). "A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells". Nature Chemical Biology. 8 (11): 890–896. doi:10.1038/nchembio.1084. PMID 23023262.
- Qi W, Chan H, Teng L, Li L, Chuai S, Zhang R, et al. (December 2012). "Selective inhibition of Ezh2 by a small molecule inhibitor blocks tumor cells proliferation". Proceedings of the National Academy of Sciences of the United States of America. 109 (52): 21360–21365. Bibcode:2012PNAS..10921360Q. doi:10.1073/pnas.1210371110. PMC 3535655. PMID 23236167.
- McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, et al. (December 2012). "EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations". Nature. 492 (7427): 108–112. Bibcode:2012Natur.492..108M. doi:10.1038/nature11606. PMID 23051747. S2CID 4385729.
- Couture JF, Hauk G, Thompson MJ, Blackburn GM, Trievel RC (July 2006). "Catalytic roles for carbon-oxygen hydrogen bonding in SET domain lysine methyltransferases". The Journal of Biological Chemistry. 281 (28): 19280–19287. doi:10.1074/jbc.M602257200. PMID 16682405.
- Epizyme Announced Clinical Data from Phase 1 Trial of EZH2 Inhibitor EPZ-6438 (E7438) to be Presented at EORTC-NCI-AACR Symposium. (2014, October 1).
- "FDA granted accelerated approval to tazemetostat for follicular lymphoma". FDA. 18 June 2020.
- Nikoloski G, Langemeijer SM, Kuiper RP, Knops R, Massop M, Tönnissen ER, et al. (August 2010). "Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes". Nature Genetics. 42 (8): 665–667. doi:10.1038/ng.620. PMID 20601954. S2CID 5814891.
- "Ensembl". Gene Tree EZH2. Retrieved February 19, 2015.
- Laible G, Wolf A, Dorn R, Reuter G, Nislow C, Lebersorger A, et al. (June 1997). "Mammalian homologues of the Polycomb-group gene Enhancer of zeste mediate gene silencing in Drosophila heterochromatin and at S. cerevisiae telomeres". The EMBO Journal. 16 (11): 3219–3232. doi:10.1093/emboj/16.11.3219. PMC 1169939. PMID 9214638.
- "NCBI UniGene". Enhancer of zeste homolog 2 (Drosophila) (EZH2). Retrieved February 1, 2015.
- "GeneCards". Enhancer Of Zeste Homolog 2 (Drosophila). Retrieved February 1, 2015.
- "Ensembl". Gene Tree EZH2. Retrieved February 1, 2015.
- Flicek P, Amode MR, Barrell D, Beal K, Billis K, Brent S, et al. (January 2014). "Ensembl 2014". Nucleic Acids Research. 42 (Database issue): D749–D755. doi:10.1093/nar/gkt1196. PMC 3964975. PMID 24316576.
Further reading
- Zeidler M, Kleer CG (September 2006). "The Polycomb group protein Enhancer of Zeste 2: its links to DNA repair and breast cancer". Journal of Molecular Histology. 37 (5–7): 219–223. doi:10.1007/s10735-006-9042-9. PMID 16855786. S2CID 2332105.
- De Haan G, Gerrits A (June 2007). "Epigenetic control of hematopoietic stem cell aging the case of Ezh2". Annals of the New York Academy of Sciences. 1106 (1): 233–239. Bibcode:2007NYASA1106..233D. doi:10.1196/annals.1392.008. PMID 17332078. S2CID 25177748.
- Hobert O, Jallal B, Ullrich A (June 1996). "Interaction of Vav with ENX-1, a putative transcriptional regulator of homeobox gene expression". Molecular and Cellular Biology. 16 (6): 3066–3073. doi:10.1128/MCB.16.6.3066. PMC 231301. PMID 8649418.
- Bonaldo MF, Lennon G, Soares MB (September 1996). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Research. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Abel KJ, Brody LC, Valdes JM, Erdos MR, McKinley DR, Castilla LH, et al. (October 1996). "Characterization of EZH1, a human homolog of Drosophila Enhancer of zeste near BRCA1". Genomics. 37 (2): 161–171. doi:10.1006/geno.1996.0537. PMID 8921387.
- Laible G, Wolf A, Dorn R, Reuter G, Nislow C, Lebersorger A, et al. (June 1997). "Mammalian homologues of the Polycomb-group gene Enhancer of zeste mediate gene silencing in Drosophila heterochromatin and at S. cerevisiae telomeres". The EMBO Journal. 16 (11): 3219–3232. doi:10.1093/emboj/16.11.3219. PMC 1169939. PMID 9214638.
- Cardoso C, Timsit S, Villard L, Khrestchatisky M, Fontès M, Colleaux L (April 1998). "Specific interaction between the XNP/ATR-X gene product and the SET domain of the human EZH2 protein". Human Molecular Genetics. 7 (4): 679–684. doi:10.1093/hmg/7.4.679. PMID 9499421.
- van Lohuizen M, Tijms M, Voncken JW, Schumacher A, Magnuson T, Wientjens E (June 1998). "Interaction of mouse polycomb-group (Pc-G) proteins Enx1 and Enx2 with Eed: indication for separate Pc-G complexes". Molecular and Cellular Biology. 18 (6): 3572–3579. doi:10.1128/MCB.18.6.3572. PMC 108938. PMID 9584197.
- Sewalt RG, van der Vlag J, Gunster MJ, Hamer KM, den Blaauwen JL, Satijn DP, et al. (June 1998). "Characterization of interactions between the mammalian polycomb-group proteins Enx1/EZH2 and EED suggests the existence of different mammalian polycomb-group protein complexes". Molecular and Cellular Biology. 18 (6): 3586–3595. doi:10.1128/mcb.18.6.3586. PMC 108940. PMID 9584199.
- Denisenko O, Shnyreva M, Suzuki H, Bomsztyk K (October 1998). "Point mutations in the WD40 domain of Eed block its interaction with Ezh2". Molecular and Cellular Biology. 18 (10): 5634–5642. doi:10.1128/MCB.18.10.5634. PMC 109149. PMID 9742080.
- van der Vlag J, Otte AP (December 1999). "Transcriptional repression mediated by the human polycomb-group protein EED involves histone deacetylation". Nature Genetics. 23 (4): 474–478. doi:10.1038/70602. PMID 10581039. S2CID 6748531.
- Cardoso C, Mignon C, Hetet G, Grandchamps B, Fontes M, Colleaux L (March 2000). "The human EZH2 gene: genomic organisation and revised mapping in 7q35 within the critical region for malignant myeloid disorders". European Journal of Human Genetics. 8 (3): 174–180. doi:10.1038/sj.ejhg.5200439. PMID 10780782.
- Raaphorst FM, Otte AP, van Kemenade FJ, Blokzijl T, Fieret E, Hamer KM, et al. (May 2001). "Distinct BMI-1 and EZH2 expression patterns in thymocytes and mature T cells suggest a role for Polycomb genes in human T cell differentiation". Journal of Immunology. 166 (10): 5925–5934. doi:10.4049/jimmunol.166.10.5925. PMID 11342607.
- O'Connell S, Wang L, Robert S, Jones CA, Saint R, Jones RS (November 2001). "Polycomblike PHD fingers mediate conserved interaction with enhancer of zeste protein". The Journal of Biological Chemistry. 276 (46): 43065–43073. doi:10.1074/jbc.M104294200. PMID 11571280.
- Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, et al. (October 2002). "The polycomb group protein EZH2 is involved in progression of prostate cancer". Nature. 419 (6907): 624–629. Bibcode:2002Natur.419..624V. doi:10.1038/nature01075. hdl:2027.42/62896. PMID 12374981. S2CID 4414767.
- Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, et al. (September 2003). "EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells". Proceedings of the National Academy of Sciences of the United States of America. 100 (20): 11606–11611. Bibcode:2003PNAS..10011606K. doi:10.1073/pnas.1933744100. PMC 208805. PMID 14500907.