Icilin
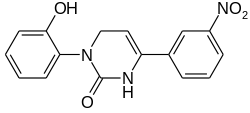
Icilin (AG-3-5) is a synthetic super-agonist of the transient receptor potential M8 (TRPM8) ion channel. Although structurally not related to menthol, it produces an extreme sensation of cold, both in humans and animals. It is almost 200 times more potent than menthol, and 2.5 times more efficacious.[1] Despite their similar effects, icilin activates the TRPM8 receptor in a different way than menthol does.[2] Icilin has been shown to be effective in the treatment of pruritus in an experimental model of itch.[3] It is now used as a research tool for the study of TRP channels, although despite its high potency it is less selective for TRPM8 over other related ion channels than are other compounds such as WS-12.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3-(2-Hydroxyphenyl)-6-(3-nitrophenyl)-3,4-dihydropyrimidin-2(1H)-one | |
| Other names
1-(2-Hydroxyphenyl)-4-(3-nitrophenyl)-3,6-dihydropyrimidin-2-one AG-3-5 | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.164.593 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H13N3O4 | |
| Molar mass | 311.29 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
References
- Wei ET, Seid DA (1983). "AG-3-5: a chemical producing sensations of cold". J. Pharm. Pharmacol. 35 (2): 110–2. doi:10.1111/j.2042-7158.1983.tb04279.x. PMID 6131976. S2CID 42844929.
- Andersson DA, Chase HW, Bevan S (2004). "TRPM8 activation by menthol, icilin, and cold is differentially modulated by intracellular pH". J. Neurosci. 24 (23): 5364–9. doi:10.1523/JNEUROSCI.0890-04.2004. PMC 6729305. PMID 15190109.
- Biró, T; Ko, MC; Bromm, B; et al. (2005). "How best to fight that nasty itch - from new insights into the neuroimmunological, neuroendocrine, and neurophysiological bases of pruritus to novel therapeutic approaches". Experimental Dermatology. 14 (3): 225–40. doi:10.1111/j.0906-6705.2005.0321a.x. PMID 15740597. S2CID 23665244.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.