Bradykinin
Bradykinin (BK) (Greek brady-, slow; -kinin, kīn(eîn) to move) is a peptide that promotes inflammation. It causes arterioles to dilate (enlarge) via the release of prostacyclin, nitric oxide, and endothelium-derived hyperpolarizing factor and makes veins constrict, via prostaglandin F2, thereby leading to leakage into capillary beds, due to the increased pressure in the capillaries. Bradykinin consists of nine amino acids, and is a physiologically and pharmacologically active peptide of the kinin group of proteins.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Bradykinin | |
| Systematic IUPAC name
(2S)-2-{(12S,32S,9S,12S,142S,17S)-11-[(2S)-2-Amino-5-(carbamimidoylamino)pentanoyl]-9-benzyl-12-(hydroxymethyl)-2,4,7,10,13,15-hexaoxo-5,8,11,16-tetraaza-1(2),3,14(1,2)-tripyrrolidina-19-benzenanonadecaphane-17-carboxamido}-5-(carbamimidoylamino)pentanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.362 |
| MeSH | Bradykinin |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C50H73N15O11 | |
| Molar mass | 1060.228 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
| kininogen 1 | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | KNG1 | ||||||
| Alt. symbols | KNG, BDK | ||||||
| NCBI gene | 3827 | ||||||
| HGNC | 6383 | ||||||
| OMIM | 612358 | ||||||
| RefSeq | NM_001102416 | ||||||
| UniProt | P01042 | ||||||
| Other data | |||||||
| Locus | Chr. 3 q21-qter | ||||||
| |||||||
| Bradykinin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Bradykinin | ||||||||
| Pfam | PF06753 | ||||||||
| InterPro | IPR009608 | ||||||||
| |||||||||
A class of drugs called angiotensin-converting-enzyme inhibitors (ACE inhibitors) increase bradykinin levels by inhibiting its degradation, thereby increasing its blood pressure lowering effect. ACE inhibitors are FDA approved for the treatment of hypertension and heart failure.
Structure
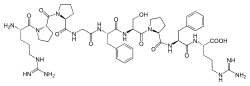
Bradykinin, sometimes referred to as BK, is a 9–amino acid peptide chain. The amino acid sequence of bradykinin is: Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg (RPPGFSPFR).[1] Its empirical formula is therefore C
50H
73N
15O
11.
Metabolism
The kinin–kallikrein system makes bradykinin by proteolytic cleavage of its kininogen precursor, high-molecular-weight kininogen (HMWK or HK), by the enzyme kallikrein. Moreover, there is compelling evidence that plasmin, a fibrinolytic enzyme, is able to generate bradykinin after HMWK cleavage.[2]
In humans, bradykinin is broken down by many different kininases: angiotensin-converting enzyme (ACE, kininase II), neprilysin,[3] NEP2, aminopeptidase P (APP), carboxypeptidase N (CPN, kininase I), Carboxypeptidase M, Neutral endopeptidase 24.15, Endothelin converting enzyme-1, Endothelin converting enzyme-2.[4]
Function
Effects
Bradykinin is a potent endothelium-dependent vasodilator and mild diuretic, which may cause a lowering of the blood pressure. It also causes contraction of non-vascular smooth muscle in the bronchus and gut, increases vascular permeability and is also involved in the mechanism of pain.[5]
During inflammation, it is released locally from mast cells and basophils during tissue damage.[6] Specifically in relation to pain, bradykinin has been shown to sensitize TRPV1 receptors, thus lowering the temperature threshold at which they activate, thus presumably contributing to allodynia.[7]
Initial secretion of bradykinin post-natally causes constriction and eventual atrophy of the ductus arteriosus, forming the ligamentum arteriosum between the pulmonary trunk and aortic arch. It also plays a role in the constriction and eventual occlusion of a number of other fetal vessels, including the umbilical arteries and vein. The differential vasoconstriction of these fetal vessels compared to the vasodilator response of other vessels suggests that the walls of these fetal vessels are different from other vessels.[8]
Receptors
- The B1 receptor (also called bradykinin receptor B1) is expressed only as a result of tissue injury, and is presumed to play a role in chronic pain. This receptor has been also described to play a role in inflammation.[9] It was shown that the kinin B1 receptor recruits neutrophil via the chemokine CXCL5 production. Moreover, endothelial cells have been described as a potential source for this B1 receptor-CXCL5 pathway.[10]
- The B2 receptor is constitutively expressed and participates in bradykinin's vasodilatory role.
The kinin B1 and B2 receptors belong to G protein coupled receptor (GPCR) family.
Disorders
Bradykinin is also thought to be the cause of the dry cough in some patients on widely prescribed angiotensin-converting enzyme (ACE) inhibitor drugs. It is thought that bradykinin is converted to inactive metabolites by ACE, therefore inhibition of this enzyme leads to increased levels of bradykinin; increased bradykinin sensitizes somatosensory fibers and thus causes hyperalgesia. Bradykinin may mediate this via pro-inflammatory peptides (e.g. substance P, neuropeptide Y) and a local release of histamine.[11][12]
In severe cases, the elevation of bradykinin may result in angioedema, a medical emergency.[13] People of African descent have up to five times increased risk of ACE inhibitor induced angioedema due to hereditary predisposing risk factors such as hereditary angioedema.[14] This refractory cough is a common cause for stopping ACE inhibitor therapy.
Overactivation of bradykinin is thought to play a role in a rare disease called hereditary angioedema.[15]
Low levels of bradykinin in the body correlate to a high body mass index in adolescents; it has been proposed that bradykinin can be used as a biomarker for metabolic syndrome.[16]
Bradykinins have been implicated in a number of cancer progression processes.[17] Increased levels of bradykinins resulting from ACE inhibitor use have been associated with increased lung cancer risks.[18] Bradykinins have been implicated in cell proliferation and migration in gastric cancers,[19] and bradykinin antagonists have been investigated as anti-cancer agents.[20]
Bradykinin has been proposed as an explanation for many symptoms associated with COVID-19, including dry coughs, myalgia, fatigue, nausea, vomiting, diarrhea, anorexia, headaches, decreased cognitive function, arrhythmia and sudden cardiac death.[21]
Therapeutic implications
A bradykinin-potentiating factor (BPF) which increases both the duration and magnitude of the effects of bradykinin on vasodilation and the consequent fall in blood pressure, was discovered in Bothrops jararaca venom.[22] On the basis of this finding, a non-protein analog of BPF which was effective orally was developed: the first angiotensin converting enzyme inhibitor captopril. It was approved by the FDA for the treatment of hypertension in 1981.{citation needed April 2019}
Currently, bradykinin inhibitors (antagonists) are being developed as potential therapies for hereditary angioedema. Icatibant is one such inhibitor. Additional bradykinin inhibitors exist. It has long been known in animal studies that bromelain, a substance obtained from the stems and leaves of the pineapple plant, suppresses trauma-induced swelling caused by the release of bradykinin into the bloodstream and tissues.[23] Other substances that act as bradykinin inhibitors include aloe[24][25] and polyphenols, substances found in red wine and green tea.[26]
History
Bradykinin was discovered in 1948 by three Brazilian physiologists and pharmacologists working at the Biological Institute, in São Paulo, Brazil, led by Dr. Maurício Rocha e Silva.[27] Together with colleagues Wilson Teixeira Beraldo and Gastão Rosenfeld, they discovered the powerful hypotensive effects of bradykinin in animal preparations. Bradykinin was detected in the blood plasma of animals after the addition of venom extracted from the Bothrops jararaca (Brazilian lancehead snake), brought by Rosenfeld from the Butantan Institute. The discovery was part of a continuing study on circulatory shock and proteolytic enzymes related to the toxicology of snake bites, started by Rocha e Silva as early as 1939. Bradykinin was to prove a new autopharmacological principle, i.e., a substance that is released in the body by a metabolic modification from precursors, which are pharmacologically active. According to B.J. Hagwood, Rocha e Silva's biographer, "The discovery of bradykinin has led to a new understanding of many physiological and pathological phenomena including circulatory shock induced by venoms and toxins." In 1957 Dr. Mauricio Rocha e Silva became full-professor at Department of Pharmacology of the Faculdade de Medicina de Ribeirão Preto of Universidade de São Paulo, in Ribeirão Preto, São Paulo, Brazil, in which he led an outstanding team of pharmacologists.
References
- Pinheiro, AS; Silbak, S; Schmaier, AH (February 2022). "Bradykinin — An elusive peptide in measuring and understanding". Research and Practice in Thrombosis and Haemostasis. 6 (2): e12673. doi:10.1002/rth2.12673. PMC 8886326. PMID 35252738.
- Marcos-Contreras OA, Martinez de Lizarrondo S, Bardou I, Orset C, Pruvost M, Anfray A, et al. (November 2016). "Hyperfibrinolysis increases blood-brain barrier permeability by a plasmin- and bradykinin-dependent mechanism". Blood. 128 (20): 2423–2434. doi:10.1182/blood-2016-03-705384. PMID 27531677.
- "MME membrane metalloendopeptidase [Homo sapiens (human)]". www.ncbi.nlm.nih.gov. Retrieved 2022-02-06.
- Campbell DJ (2018-09-19). "Neprilysin Inhibitors and Bradykinin". Frontiers in Medicine. 5: 257. doi:10.3389/fmed.2018.00257. PMC 6157573. PMID 30283782.
- Mutschler E, Schäfer-Korting M (1997). Arzneimittelwirkungen (in German) (7 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. ISBN 978-3-8047-1377-2.
- Dray A, Perkins M (March 1993). "Bradykinin and inflammatory pain". Trends in Neurosciences. 16 (3): 99–104. doi:10.1016/0166-2236(93)90133-7. PMID 7681240. S2CID 4061940.
- Mathivanan S, Devesa I, Changeux JP, Ferrer-Montiel A (2016-06-23). "Bradykinin Induces TRPV1 Exocytotic Recruitment in Peptidergic Nociceptors". Frontiers in Pharmacology. 7: 178. doi:10.3389/fphar.2016.00178. PMC 4917537. PMID 27445816.
- Standring S, Gray H (2016). "Ch. 52: Development of the thorax. Section: Changes in the Fetal Circulation and Occlusion of Fetal Vessels after Birth". Gray's Anatomy: The Anatomical Basis of Clinical Practice (41st ed.). Elsevier. pp. 905–930. ISBN 9780702052309. OCLC 920806541.
- McLean PG, Ahluwalia A, Perretti M (August 2000). "Association between kinin B(1) receptor expression and leukocyte trafficking across mouse mesenteric postcapillary venules". The Journal of Experimental Medicine. 192 (3): 367–80. doi:10.1084/jem.192.3.367. PMC 2193221. PMID 10934225.
- Duchene J, Lecomte F, Ahmed S, Cayla C, Pesquero J, Bader M, et al. (October 2007). "A novel inflammatory pathway involved in leukocyte recruitment: role for the kinin B1 receptor and the chemokine CXCL5". Journal of Immunology. 179 (7): 4849–56. doi:10.4049/jimmunol.179.7.4849. PMC 3696729. PMID 17878384.
- Fox AJ, Lalloo UG, Belvisi MG, Bernareggi M, Chung KF, Barnes PJ (July 1996). "Bradykinin-evoked sensitization of airway sensory nerves: a mechanism for ACE-inhibitor cough". Nature Medicine. 2 (7): 814–7. doi:10.1038/nm0796-814. PMID 8673930. S2CID 6040673.
- Karlberg BE (April 1993). "Cough and inhibition of the renin-angiotensin system". Journal of Hypertension Supplement. 11 (3): S49-52. PMID 8315520.
- Li HH (2018-05-22). "Angioedema: Practice Essentials, Background, Pathophysiology". Medscape.
- Guyer AC, Banerji A. "ACE inhibitor-induced angioedema". UpToDate. Retrieved 2018-06-03.
- Bas M, Adams V, Suvorava T, Niehues T, Hoffmann TK, Kojda G (August 2007). "Nonallergic angioedema: role of bradykinin". Allergy. 62 (8): 842–56. doi:10.1111/j.1398-9995.2007.01427.x. PMID 17620062. S2CID 22772933.
- Sugawara, Akira; Shimada, Hiroki; Otsubo, Yuri; Kouketsu, Takumi; Suzuki, Susumu; Yokoyama, Atsushi (2021-05-27). "The usefulness of angiotensin-(1-7) and des-Arg9-bradykinin as novel biomarkers for metabolic syndrome". Hypertension Research. 44 (8): 1034–1036. doi:10.1038/s41440-021-00671-9. ISSN 1348-4214. PMID 34045691. S2CID 235206108.
- Stewart JM, Gera L, Chan DC, Bunn PA, York EJ, Simkeviciene V, Helfrich B (April 2002). "Bradykinin-related compounds as new drugs for cancer and inflammation". Canadian Journal of Physiology and Pharmacology. 80 (4): 275–80. doi:10.1139/y02-030. PMID 12025961.
- Kmietowicz Z (October 2018). "ACE inhibitors are linked to increased lung cancer risk, study finds". BMJ. 363: k4471. doi:10.1136/bmj.k4471. PMID 30355572. S2CID 53024740.
- Wang G, Sun J, Liu G, Fu Y, Zhang X (December 2017). "Bradykinin Promotes Cell Proliferation, Migration, Invasion, and Tumor Growth of Gastric Cancer Through ERK Signaling Pathway". Journal of Cellular Biochemistry. 118 (12): 4444–4453. doi:10.1002/jcb.26100. PMID 28464378. S2CID 3954713.
- Stewart JM (2003). "Bradykinin antagonists as anti-cancer agents". Current Pharmaceutical Design. 9 (25): 2036–42. doi:10.2174/1381612033454171. PMID 14529414.
- Garvin MR, Alvarez C, Miller JI, Prates ET, Walker AM, Amos BK, et al. (July 7, 2020). "A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm". eLife. 9. doi:10.7554/eLife.59177. PMC 7410499. PMID 32633718.
- Ferreira S (1965). "A bradykinin-potentiation factor (BPF) present in the venom of Bothrops jararaca". British Journal of Pharmacology and Chemotherapy. 24 (1): 163–69. doi:10.1111/j.1476-5381.1965.tb02091.x. PMC 1704050. PMID 14302350.
- Lotz-Winter H (June 1990). "On the pharmacology of bromelain: an update with special regard to animal studies on dose-dependent effects". Planta Medica. 56 (3): 249–53. doi:10.1055/s-2006-960949. PMID 2203073.
- Bautista-Pérez R, Segura-Cobos D, Vázquez-Cruz B (July 2004). "In vitro antibradykinin activity of Aloe barbadensis gel". Journal of Ethnopharmacology. 93 (1): 89–92. doi:10.1016/j.jep.2004.03.030. PMID 15182910.
- Yagi A, Harada N, Yamada H, Iwadare S, Nishioka I (October 1982). "Antibradykinin active material in Aloe saponaria". Journal of Pharmaceutical Sciences. 71 (10): 1172–4. doi:10.1002/jps.2600711024. PMID 7143219.
- Richard T, Delaunay JC, Mérillon JM, Monti JP (December 2003). "Is the C-terminal region of bradykinin the binding site of polyphenols?". Journal of Biomolecular Structure & Dynamics. 21 (3): 379–85. doi:10.1080/07391102.2003.10506933. PMID 14616033. S2CID 24807751.
- Rocha e Silva M, Beraldo WT, Rosenfeld G (1949). "Bradykinin, a hypotensive and smooth muscle stimulating factor released from plasma globulin by snake venoms and by trypsin". American Journal of Physiology. 156 (2): 261–73. doi:10.1152/ajplegacy.1949.156.2.261. PMID 18127230.